Allergy Asthma Immunol Res.
2018 Sep;10(5):543-554. 10.4168/aair.2018.10.5.543.
Bcl11b Regulates IL-17 Through the TGF-β/Smad Pathway in HDM-Induced Asthma
- Affiliations
-
- 1Department of Immunology, Shenzhen University School of Medicine, Shenzhen, China. chensi@szu.edu.cn
- 2Department of Pulmonary Medicine, The First Affiliated Hospital of Shenzhen University, Shenzhen, China.
- KMID: 2418051
- DOI: http://doi.org/10.4168/aair.2018.10.5.543
Abstract
- PURPOSE
T helper (Th) 17 cells play a critical role in the development of asthma, but the underlying mechanism of how interleukin (IL)-17 is regulated in allergic airway inflammation is poorly understood. In this study, we investigated the impact of Bcl11b on Th17 response in asthma.
METHODS
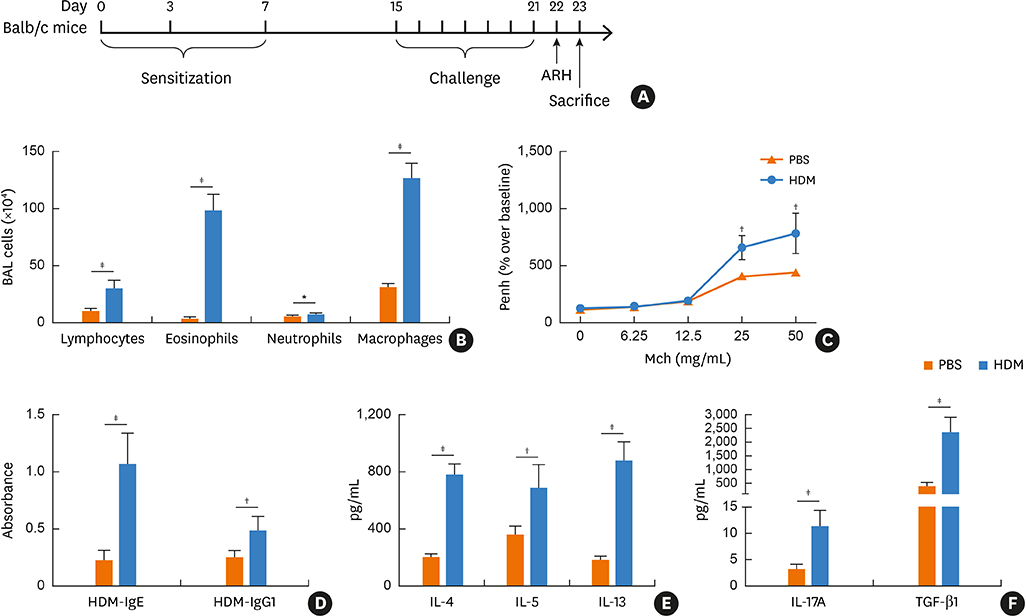
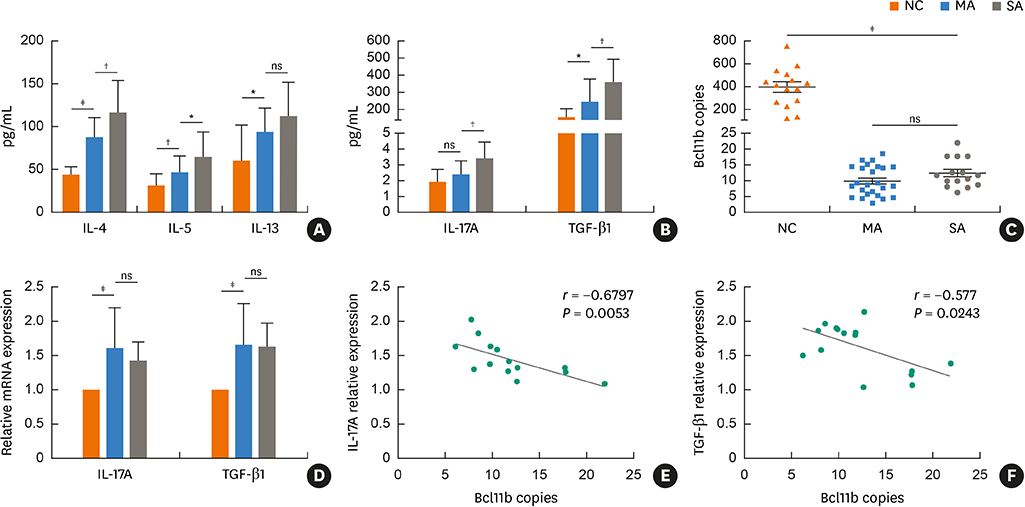
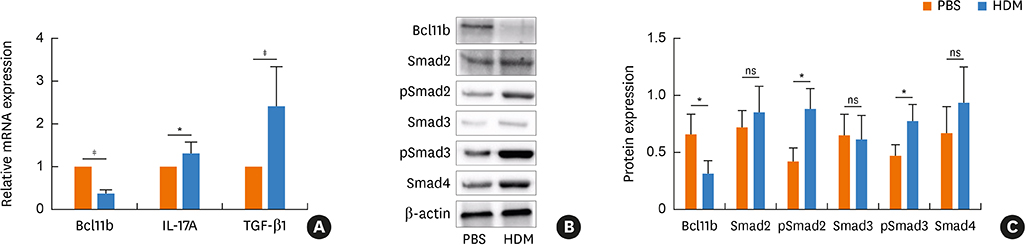
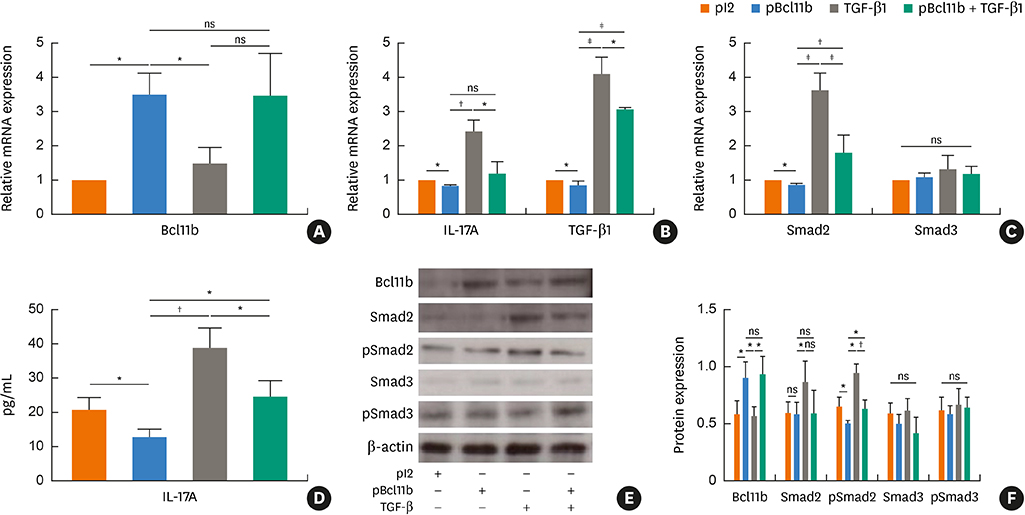
Blood samples from patients with mild asthma (MA) and severe asthma (SA) were collected. Expression of Bcl11b, IL-4, IL-5, IL-13, IL-17A and transforming growth factor (TGF)-β1 were determined in CD4+ T cells and plasma by polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA). Relative mRNA and protein levels of Bcl11b, IL-17A and genes involved in the TGF/Smad signaling pathway were examined by PCR, ELISA and western blot analysis in house dust mite (HDM)-challenged mice. Ectopic expression of Bcl11b in HDM-stimulated primary mouse splenocytes was achieved by nucleofection of Bcl11b expression plasmid.
RESULTS
We found significantly decreased Bcl11b but increased IL-17A and TGF-β1 expression in patients with asthma and a strongly negative correlation between Bcl11b and these 2 cytokines in SA patients. Similar expression patterns of Bcl11b, IL-17A and TGF-β1 were also found in mice with HDM-induced allergic airway inflammation. We demonstrated further that Smad2/3 phosphorylation was increased in HDM-challenged mice and that ectopic expression of Bcl11b in HDM-stimulated primary mouse splenocytes reduced Smad2 phosphorylation and IL-17 expression.
CONCLUSIONS
Our findings demonstrate a potential effect of Bc111b in controlling IL-17-mediated inflammation in asthma and suggest that Bc111b may be a useful therapeutic target for asthma.
MeSH Terms
-
Animals
Asthma*
Blotting, Western
Cytokines
Ectopic Gene Expression
Enzyme-Linked Immunosorbent Assay
Humans
Inflammation
Interleukin-13
Interleukin-17*
Interleukin-4
Interleukin-5
Interleukins
Mice
Phosphorylation
Plasma
Plasmids
Polymerase Chain Reaction
Pyroglyphidae
RNA, Messenger
T-Lymphocytes
Transforming Growth Factors
Cytokines
Interleukin-13
Interleukin-17
Interleukin-4
Interleukin-5
Interleukins
RNA, Messenger
Transforming Growth Factors
Figure
Reference
-
1. Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015; 349:1106–1110.
Article2. Choi JP, Lee SM, Choi HI, Kim MH, Jeon SG, Jang MH, et al. House dust mite-derived chitin enhances Th2 cell response to inhaled allergens, mainly via a TNF-α-dependent pathway. Allergy Asthma Immunol Res. 2016; 8:362–374.
Article3. Wypych TP, Marzi R, Wu GF, Lanzavecchia A, Sallusto F. Role of B cells in TH cell responses in a mouse model of asthma. J Allergy Clin Immunol. 2017; 6749:31430–31436.
Article4. Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev. 2017; 278:162–172.
Article5. Newcomb DC, Peebles RS Jr. Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013; 25:755–760.
Article6. Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001; 108:430–438.
Article7. Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009; 123:1185–1187.
Article8. Ito T, Hirose K, Norimoto A, Tamachi T, Yokota M, Saku A, et al. Dectin-1 plays an important role in house dust mite-induced allergic airway inflammation through the activation of CD11b+ dendritic cells. J Immunol. 2017; 198:61–70.
Article9. Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010; 329:89–93.
Article10. Wakabayashi Y, Watanabe H, Inoue J, Takeda N, Sakata J, Mishima Y, et al. Bcl11b is required for differentiation and survival of alphabeta T lymphocytes. Nat Immunol. 2003; 4:533–539.11. Albu DI, Feng D, Bhattacharya D, Jenkins NA, Copeland NG, Liu P, et al. Bcl11b is required for positive selection and survival of double-positive thymocytes. J Exp Med. 2007; 204:3003–3015.
Article12. Zhang S, Rozell M, Verma RK, Albu DI, Califano D, VanValkenburgh J, et al. Antigen-specific clonal expansion and cytolytic effector function of CD8+ T lymphocytes depend on the transcription factor Bcl11b. J Exp Med. 2010; 207:1687–1699.
Article13. Vanvalkenburgh J, Albu DI, Bapanpally C, Casanova S, Califano D, Jones DM, et al. Critical role of Bcl11b in suppressor function of T regulatory cells and prevention of inflammatory bowel disease. J Exp Med. 2011; 208:2069–2081.
Article14. Wang Z, Zhang LJ, Guha G, Li S, Kyrylkova K, Kioussi C, et al. Selective ablation of Ctip2/Bcl11b in epidermal keratinocytes triggers atopic dermatitis-like skin inflammatory responses in adult mice. PLoS One. 2012; 7:e51262.
Article15. Califano D, Sweeney KJ, Le H, VanValkenburgh J, Yager E, O'Connor W Jr, et al. Diverting T helper cell trafficking through increased plasticity attenuates autoimmune encephalomyelitis. J Clin Invest. 2014; 124:174–187.
Article16. Chen S, Huang X, Chen S, Yang L, Shen Q, Zheng H, et al. The role of Bcl11b in regulating the proliferation of human naive T cells. Hum Immunol. 2012; 73:456–464.
Article17. Wang H, Lin J, Zeng L, Ouyang C, Ran P, Yang P, et al. Der f 31, a novel allergen from Dermatophagoides farinae, activates epithelial cells and enhances lung-resident group 2 innate lymphoid cells. Sci Rep. 2017; 7:8519.
Article18. Lv Q, Yang XM, Xiao XJ, Chen S, Yang PC, Liu ZG, et al. Percentage of Th17 cells in spleen and IL-17 level in bronchoalveolar lavage fluid in dermatophagoides farinae allergic asthma mice. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2015; 33:127–129.19. Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002; 57:643–648.
Article20. Park SJ, Lee YC. Interleukin-17 regulation: an attractive therapeutic approach for asthma. Respir Res. 2010; 11:78.
Article21. Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009; 123:1004–1011.
Article22. Stockinger B, Omenetti S. The dichotomous nature of T helper 17 cells. Nat Rev Immunol. 2017; 17:535–544.
Article23. Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci. 2008; 1143:188–211.
Article24. Jin Y, Deng Z, Cao C, Li L. IL-17 polymorphisms and asthma risk: a meta-analysis of 11 single nucleotide polymorphisms. J Asthma. 2015; 52:981–988.
Article25. Nakagome K, Matsushita S, Nagata M. Neutrophilic inflammation in severe asthma. Int Arch Allergy Immunol. 2012; 158:Suppl 1. 96–102.
Article26. Cismasiu VB, Adamo K, Gecewicz J, Duque J, Lin Q, Avram D. Bcl11b functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter. Oncogene. 2005; 24:6753–6764.
Article27. Zhang LJ, Vogel WK, Liu X, Topark-Ngarm A, Arbogast BL, Maier CS, et al. Coordinated regulation of transcription factor Bcl11b activity in thymocytes by the mitogen-activated protein kinase (MAPK) pathways and protein sumoylation. J Biol Chem. 2012; 287:26971–26988.
Article28. Kueh HY, Yui MA, Ng KK, Pease SS, Zhang JA, Damle SS, et al. Asynchronous combinatorial action of four regulatory factors activates Bcl11b for T cell commitment. Nat Immunol. 2016; 17:956–965.
Article29. Ebel ME, Kansas GS. Functions of Smad transcription factors in TGF-β1-induced selectin ligand expression on murine CD4 Th cells. J Immunol. 2016; 197:2627–2634.
Article30. Groneberg DA, Witt H, Adcock IM, Hansen G, Springer J. Smads as intracellular mediators of airway inflammation. Exp Lung Res. 2004; 30:223–250.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low Level Light Therapy Using an 830-nm Light Emitting Diode Promotes Wound Healing via TGF-β/SMAD Pathway Activation
- The Role of Tripartite Motif Family Proteins in TGF-β Signaling Pathway and Cancer
- TGF-β induces Smad2 Phosphorylation, ARE Induction, and Trophoblast Differentiation
- TGF-beta-activated kinase-1: New insights into the mechanism of TGF-beta signaling and kidney disease
- Inhibitory Effects of Resveratrol on Airway Remodeling by Transforming Growth Factor-β/Smad Signaling Pathway in Chronic Asthma Model