Allergy Asthma Immunol Res.
2017 Jan;9(1):25-34. 10.4168/aair.2017.9.1.25.
Inhibitory Effects of Resveratrol on Airway Remodeling by Transforming Growth Factor-β/Smad Signaling Pathway in Chronic Asthma Model
- Affiliations
-
- 1Division of Allergy, Pulmonary and Critical Care Medicine, Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea. chinkook@catholic.ac.kr sooklee@catholic.ac.kr
- KMID: 2355891
- DOI: http://doi.org/10.4168/aair.2017.9.1.25
Abstract
- PURPOSE
Asthma is a chronic airway disease characterized by airway remodeling, leading to a progressive decline in lung function. Therapeutic agents that attenuate airway remodeling can complement the limited effects of traditional glucocorticoids. In this study, we investigated the effect of resveratrol on allergic airway inflammation and remodeling in a murine model of chronic bronchial asthma.
METHODS
Peribronchial smooth muscle thickening that developed in mice challenged with a 3-month repeated exposure to ovalbumin (OVA) was used to study airway remodeling. Oral resveratrol was administered daily during the OVA challenge. The expression of TGF-β1/Smad signaling proteins and downstream mesenchymal markers in the presence or absence of resveratrol was examined in bronchial epithelial cells.
RESULTS
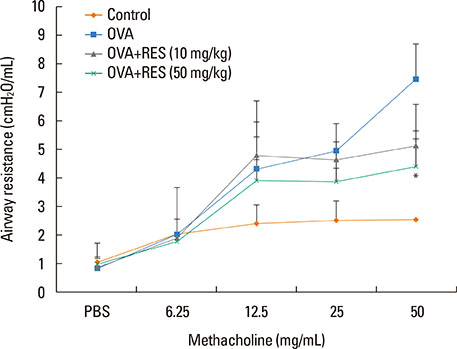
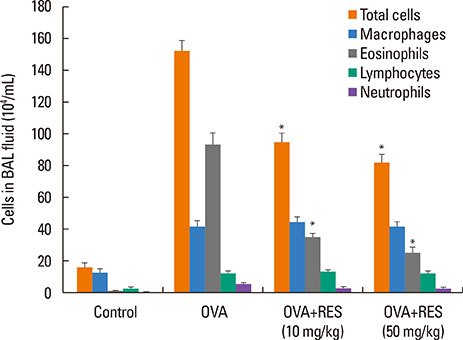
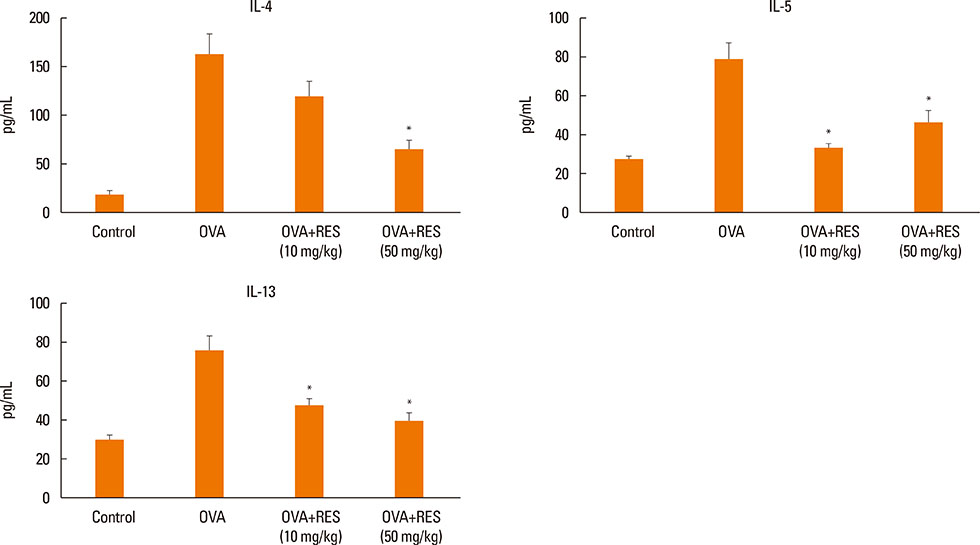
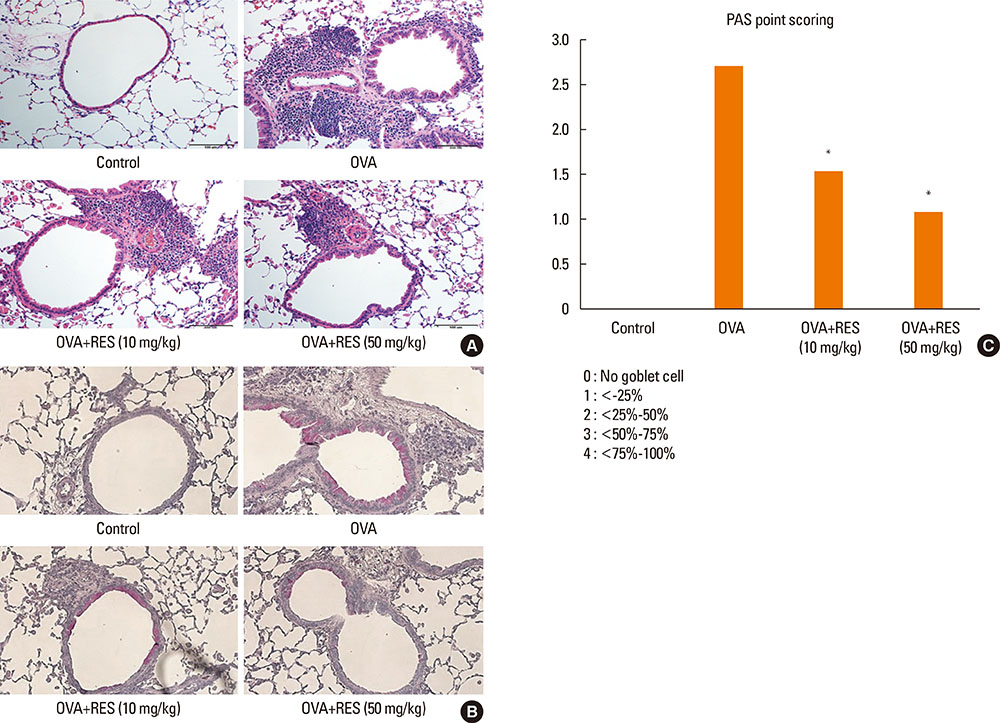
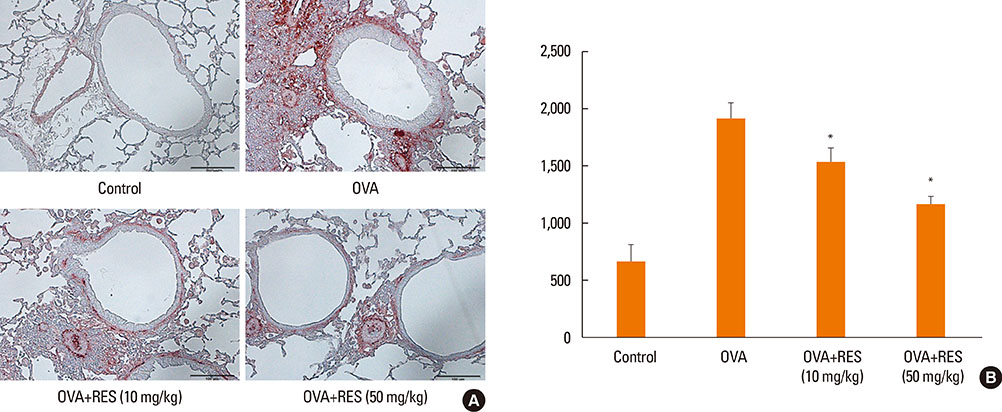
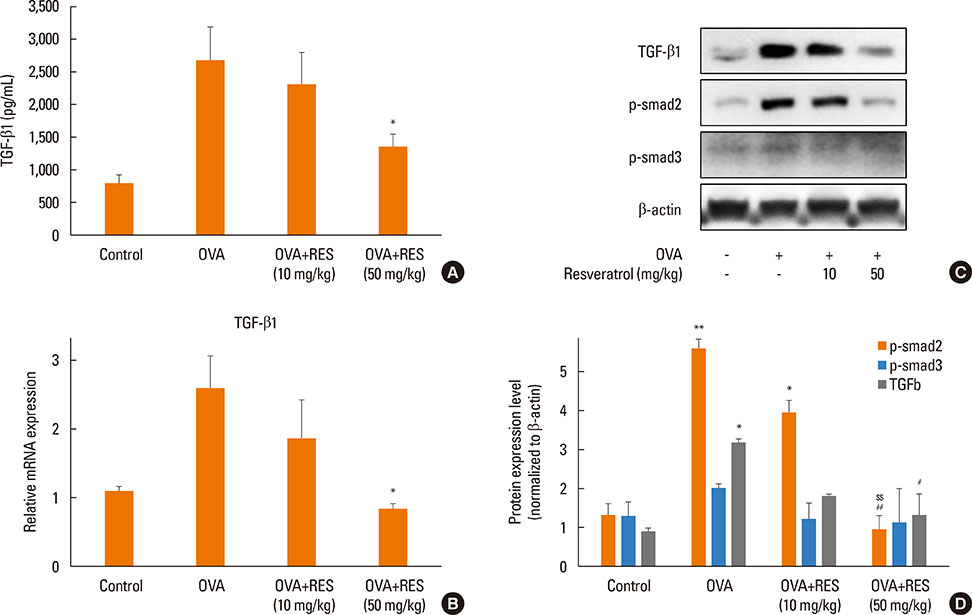
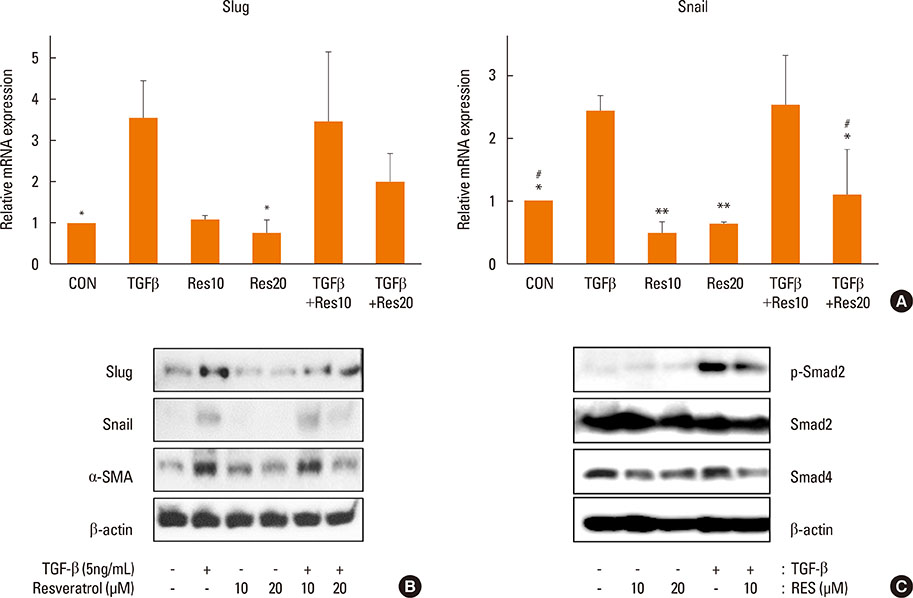
OVA sensitization and chronic challenge increased airway hyperresponsiveness, inflammation, goblet cell hyperplasia, α-smooth muscle actin (SMA), and collagen deposition. Resveratrol effectively suppressed OVA-induced airway inflammation and remodeling. The expression of TGF-β1/phosphorylated Smad2/3 was increased in the lung tissues of OVA-challenged mice but effectively inhibited by resveratrol. In bronchial epithelial cells, the TGF-β1-induced expression of the mesenchymal markers snail, slug, vimentin, and α-SMA was suppressed by resveratrol treatment.
CONCLUSIONS
Resveratrol effectively ameliorated both airway inflammation and airway structural changes in a mouse model of bronchial asthma. These effects were mediated by decreased TGF-β1 expression, in turn suppressing TGF-β1/Smad signaling and the epithelial-mesenchymal transition process. Our results demonstrate the potential benefits of resveratrol for the treatment of airway remodeling associated with bronchial asthma.
Keyword
MeSH Terms
-
Actins
Airway Remodeling*
Animals
Asthma*
Collagen
Complement System Proteins
Epithelial Cells
Epithelial-Mesenchymal Transition
Gastropoda
Glucocorticoids
Goblet Cells
Hyperplasia
Inflammation
Lung
Mice
Muscle, Smooth
Ovalbumin
Ovum
Snails
Vimentin
Actins
Collagen
Complement System Proteins
Glucocorticoids
Ovalbumin
Vimentin
Figure
Cited by 1 articles
-
TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model
Joon Young Choi, Hwa Young Lee, Jung Hur, Kyung Hoon Kim, Ji Young Kang, Chin Kook Rhee, Sook Young Lee
Allergy Asthma Immunol Res. 2018;10(3):216-224. doi: 10.4168/aair.2018.10.3.216.
Reference
-
1. Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003; 111:291–297.2. Pain M, Bermudez O, Lacoste P, Royer PJ, Botturi K, Tissot A, et al. Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur Respir Rev. 2014; 23:118–130.3. Durrani SR, Viswanathan RK, Busse WW. What effect does asthma treatment have on airway remodeling? Current perspectives. J Allergy Clin Immunol. 2011; 128:439–448.4. Manuyakorn W, Howarth PH, Holgate ST. Airway remodelling in asthma and novel therapy. Asian Pac J Allergy Immunol. 2013; 31:3–10.5. Usmani OS. Small airways dysfunction in asthma: evaluation and management to improve asthma control. Allergy Asthma Immunol Res. 2014; 6:376–388.6. Sposato B, Scalese M, Migliorini MG, Di Tomassi M, Scala R. Small airway impairment and bronchial hyperresponsiveness in asthma onset. Allergy Asthma Immunol Res. 2014; 6:242–251.7. Makino S, Sagara H. Evolution of asthma concept and effect of current asthma management guidelines. Allergy Asthma Immunol Res. 2010; 2:172–176.8. Chang WS, Kim EJ, Lim YM, Yoon D, Son JY, Park JW, et al. Age-related changes in immunological factors and their relevance in allergic disease development during childhood. Allergy Asthma Immunol Res. 2016; 8:338–345.9. Chen Y, Wong GW, Li J. Environmental exposure and genetic predisposition as risk factors for asthma in China. Allergy Asthma Immunol Res. 2016; 8:92–100.10. Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005; 233:706–720.11. Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014; 69:760–765.12. Hackett TL. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol. 2012; 12:53–59.13. Wood LG, Wark PA, Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal. 2010; 13:1535–1548.14. Royce SG, Dang W, Yuan G, Tran J, El Osta A, Karagiannis TC, et al. Resveratrol has protective effects against airway remodeling and airway hyperreactivity in a murine model of allergic airways disease. Pathobiol Aging Age Relat Dis. 2011; 1:7134.15. Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009; 9:418–424.16. Wang H, Zhang H, Tang L, Chen H, Wu C, Zhao M, et al. Resveratrol inhibits TGF-beta1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology. 2013; 303:139–146.17. Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, et al. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015; 15:97.18. Xiao Z, Chen C, Meng T, Zhang W, Zhou Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-beta pathway on matrix metalloproteinase 7. Exp Biol Med (Maywood). 2016; 241:140–146.19. Chen M, Lv Z, Jiang S. The effects of triptolide on airway remodelling and transforming growth factor-beta(1)/Smad signalling pathway in ovalbumin-sensitized mice. Immunology. 2011; 132:376–384.20. Xiong YY, Wang JS, Wu FH, Li J, Kong LY. The effects of (+/-)-Praeruptorin A on airway inflammation, remodeling and transforming growth factor-beta1/Smad signaling pathway in a murine model of allergic asthma. Int Immunopharmacol. 2012; 14:392–400.21. Lin CH, Shen ML, Kao ST, Wu DC. The effect of sesamin on airway fibrosis in vitro and in vivo. Int Immunopharmacol. 2014; 22:141–150.22. Tarkowski M, Vanoirbeek JA, Vanhooren HM, De Vooght V, Mercier CM, Ceuppens J, et al. Immunological determinants of ventilatory changes induced in mice by dermal sensitization and respiratory challenge with toluene diisocyanate. Am J Physiol Lung Cell Mol Physiol. 2007; 292:L207–L214.23. Kruger NJ. The Bradford method for protein quantitation. Methods Mol Biol. 1994; 32:9–15.24. Padrid P, Snook S, Finucane T, Shiue P, Cozzi P, Solway J, et al. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am J Respir Crit Care Med. 1995; 151:184–193.25. Rhee CK, Kim JW, Park CK, Kim JS, Kang JY, Kim SJ, et al. Effect of imatinib on airway smooth muscle thickening in a murine model of chronic asthma. Int Arch Allergy Immunol. 2011; 155:243–251.26. Rhee CK, Kang JY, Park CK, Lee SY, Kwon SS, Kim YK, et al. Effect of nilotinib on airway remodeling in a murine model of chronic asthma. Exp Lung Res. 2014; 40:199–210.27. Royce SG, Tang ML. The effects of current therapies on airway remodeling in asthma and new possibilities for treatment and prevention. Curr Mol Pharmacol. 2009; 2:169–181.28. Groneberg DA, Witt H, Adcock IM, Hansen G, Springer J. Smads as intracellular mediators of airway inflammation. Exp Lung Res. 2004; 30:223–250.29. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003; 425:577–584.30. Qu ZH, Yang ZC, Chen L, Lv ZD, Yi MJ, Ran N. Inhibition airway remodeling and transforming growth factor-beta1/Smad signaling pathway by astragalus extract in asthmatic mice. Int J Mol Med. 2012; 29:564–568.31. Yang ZC, Yi MJ, Ran N, Wang C, Fu P, Feng XY, et al. Transforming growth factorbeta1 induces bronchial epithelial cells to mesenchymal transition by activating the Snail pathway and promotes airway remodeling in asthma. Mol Med Rep. 2013; 8:1663–1668.32. Gong JH, Cho IH, Shin D, Han SY, Park SH, Kang YH. Inhibition of airway epithelial-to-mesenchymal transition and fibrosis by kaempferol in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab Invest. 2014; 94:297–308.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Tripartite Motif Family Proteins in TGF-β Signaling Pathway and Cancer
- The Wnt/beta-catenin signaling pathway regulates the development of airway remodeling in patients with asthma
- TGF-beta-activated kinase-1: New insights into the mechanism of TGF-beta signaling and kidney disease
- bFGF & FGFR Expression in Chronic Airway Remodeling of Asthma
- Low Level Light Therapy Using an 830-nm Light Emitting Diode Promotes Wound Healing via TGF-β/SMAD Pathway Activation