Nutr Res Pract.
2015 Dec;9(6):619-627. 10.4162/nrp.2015.9.6.619.
Effects of natural raw meal (NRM) on high-fat diet and dextran sulfate sodium (DSS)-induced ulcerative colitis in C57BL/6J mice
- Affiliations
-
- 1Department of Food Science and Nutrition, and Kimchi Research Institute, Pusan National University, 2, Busandaehak-ro 63 beon-gil, Geumjeong-gu, Busan 609-735, Korea. kunypark@pusan.ac.kr
- 2Erom R&D Center, Erom Co., Ltd., Chuncheon, Gangwon-do 200-943, Korea.
- KMID: 2313886
- DOI: http://doi.org/10.4162/nrp.2015.9.6.619
Abstract
- BACKGROUND/OBJECTIVES
Colitis is a serious health problem, and chronic obesity is associated with the progression of colitis. The aim of this study was to determine the effects of natural raw meal (NRM) on high-fat diet (HFD, 45%) and dextran sulfate sodium (DSS, 2% w/v)-induced colitis in C57BL/6J mice.
MATERIALS/METHODS
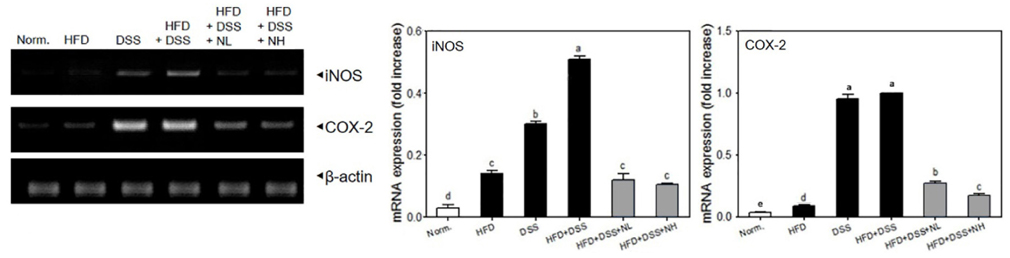
Body weight, colon length, and colon weight-to-length ratio, were measured directly. Serum levels of obesity-related biomarkers, triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), insulin, leptin, and adiponectin were determined using commercial kits. Serum levels of pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-alpha), interleukin (IL)-1beta, and IL-6 were detected using a commercial ELISA kit. Histological study was performed using a hematoxylin and eosin (H&E) staining assay. Colonic mRNA expressions of TNF-alpha, IL-1beta, IL-6, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) were determined by RT-PCR assay.
RESULTS
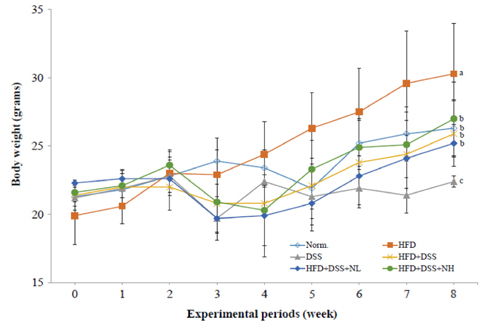
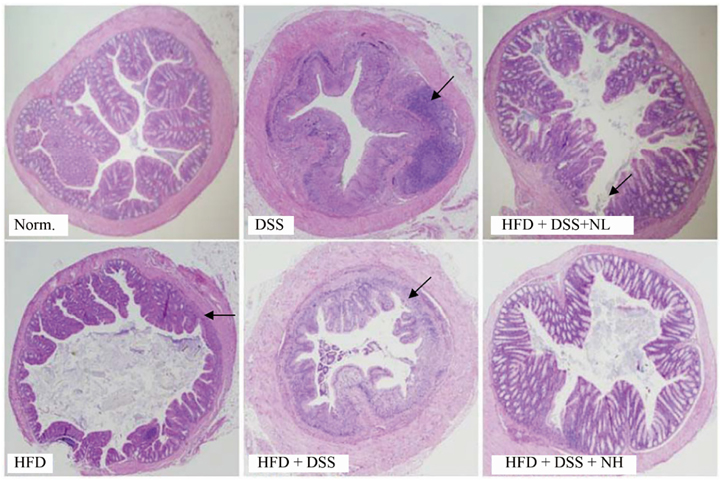
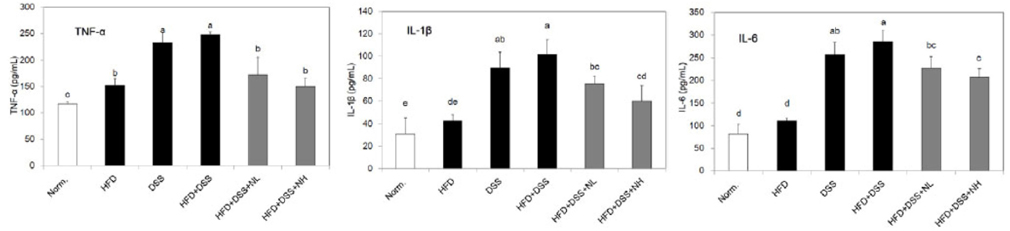
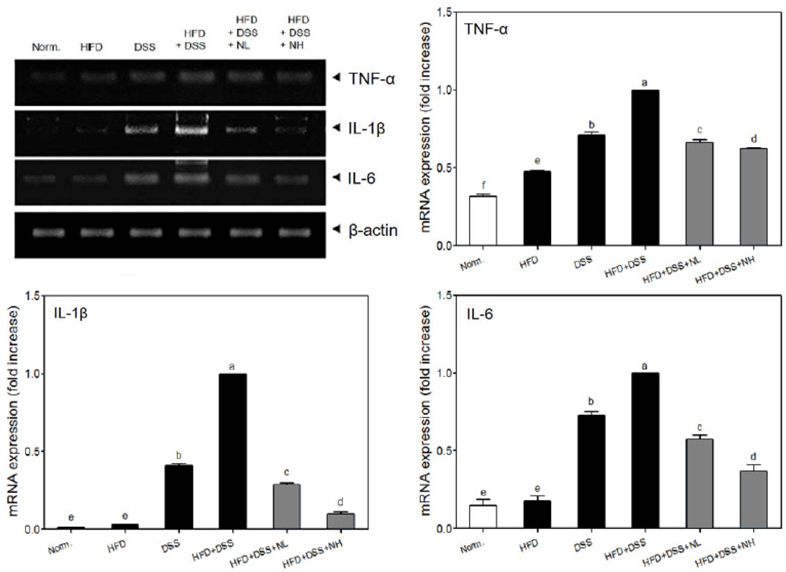
Body weight and obesity-related biomarkers (TG, TC, LDL, HDL, insulin, leptin, and adiponectin) were regulated and obesity was prevented in NRM treated mice. NRM significantly suppressed colon shortening and reduced colon weight-to-length ratio in HFD+DSS induced colitis in C57BL/6J mice (P < 0.05). Histological observations suggested that NRM reduced edema, mucosal damage, and the loss of crypts induced by HFD and DSS. In addition, NRM decreased the serum levels of pro-inflammatory cytokines, TNF-alpha, IL-1beta, and IL-6 and inhibited the mRNA expressions of these cytokines, and iNOS and COX-2 in colon mucosa (P < 0.05).
CONCLUSION
The results suggest that NRM has an anti-inflammatory effect against HFD and DSS-induced colitis in mice, and that these effects are due to the amelioration of HFD and/or DSS-induced inflammatory reactions.
Keyword
MeSH Terms
-
Adiponectin
Animals
Biomarkers
Body Weight
Cholesterol
Colitis
Colitis, Ulcerative*
Colon
Cyclooxygenase 2
Cytokines
Dextran Sulfate*
Dextrans*
Diet, High-Fat*
Edema
Enzyme-Linked Immunosorbent Assay
Eosine Yellowish-(YS)
Hematoxylin
Insulin
Interleukin-6
Interleukins
Leptin
Lipoproteins
Meals*
Mice*
Mucous Membrane
Nitric Oxide Synthase Type II
Obesity
RNA, Messenger
Triglycerides
Tumor Necrosis Factor-alpha
Ulcer*
Adiponectin
Cholesterol
Cyclooxygenase 2
Cytokines
Dextran Sulfate
Dextrans
Eosine Yellowish-(YS)
Hematoxylin
Insulin
Interleukin-6
Interleukins
Leptin
Lipoproteins
Nitric Oxide Synthase Type II
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Song M, Kim H, Suh H. Analysis of Korea national plant used on temple food. J Korean Soc Jungshin Sci. 2005; 9:1–16.2. Lee JR. A study on Buddhist monk's dietary life in relation to the contemporary culture of well-being. J Indian Philos. 2013; 38:57–79.3. Chung SS, Jan YS. Consumer's recognition, nutrient composition, and safety evaluation of commercial Sunsik and Saengsik. Korean J Food Cult. 2003; 18:235–243.4. Kim HY, Kim JH, Lee SA, Chang HE, Park MH, Hwang SJ, Lee JY, Mok C, Hong SG. Saengshik, a formulated health food, prevents liver damage in CCl4-induced mice and increases antioxidant activity in elderly women. J Med Food. 2008; 11:323–330.
Article5. Kim M, Kim ES, Park MH, Hwang SJ, Jeong Y. Saengshik, a formulated health food, decreases blood glucose and increases survival rate in streptozotocin-induced diabetic rats. J Med Food. 2004; 7:162–167.
Article6. Kang SM, Shim JY, Hwang SJ, Hong S, Jang HE, Park MH. Effects of Saengshik supplementation on health improvement in diet-induced hypercholesterolemic rats. J Korean Soc Food Sci Nutr. 2003; 32:906–912.
Article7. Ha TY, Kim NY. The effects of uncooked grains and vegetables with mainly brown rice on weight control and serum components in Korean overweight/obese female. Korean J Nutr. 2003; 36:183–190.8. Song MK, Hong SG, Hwang SJ, Park OJ, Park MH. Improve effects of Saengshik on patient with fatty liver and hyperlipidemia in murine. Korean J Nutr. 2003; 36:834–840.9. Lim BO, Jeong YJ, Park MH, Kim JD, Hwang SJ, Yu BP. Immunoregulatory effects of Saengshik on DSS-induced inflammatory bowel disease in mouse model system. J Korean Soc Food Sci Nutr. 2007; 36:32–42.
Article10. Lee YJ, Lee HM, Park TS. Effects of uncooked powdered food on antioxidative system and serum mineral concentrations in rats fed unbalanced diet. Korean J Nutr. 2003; 36:898–907.11. Oh YJ, Kim JM, Song SW, Ha HC, Kim HS. Protective effect of Saengshik supplementation on lead induced toxicity in rats. J Korean Soc Food Sci Nutr. 2005; 34:959–967.
Article12. Klemans RJ, Knol EF, Michelsen-Huisman A, Pasmans SG, de Kruijf-Broekman W, Bruijnzeel-Koomen CA, van Hoffen E, Knulst AC. Components in soy allergy diagnostics: Gly m 2S albumin has the best diagnostic value in adults. Allergy. 2013; 68:1396–1402.
Article13. Abu-Abid S, Szold A, Klausner J. Obesity and cancer. J Med. 2002; 33:73–86.14. Gaesser GA. Carbohydrate quantity and quality in relation to body mass index. J Am Diet Assoc. 2007; 107:1768–1780.
Article15. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012; 307:491–497.
Article16. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007; 86:556–565.
Article17. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009; 15:914–920.
Article18. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007; 369:1627–1640.
Article19. Batra A, Okur B, Glauben R, Erben U, Ihbe J, Stroh T, Fedke I, Chang HD, Zeitz M, Siegmund B. Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology. 2010; 151:56–62.
Article20. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009; 361:2066–2078.
Article21. Rovedatti L, Kudo T, Biancheri P, Sarra M, Knowles CH, Rampton DS, Corazza GR, Monteleone G, Di Sabatino A, Macdonald TT. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009; 58:1629–1636.
Article22. Mudter J, Neurath MF. IL-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007; 13:1016–1023.
Article23. Teixeira LG, Leonel AJ, Aguilar EC, Batista NV, Alves AC, Coimbra CC, Ferreira AV, de Faria AM, Cara DC, Alvarez Leite JI. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011; 10:204.
Article24. Park J, Yang M, Jun HS, Lee JH, Bae HK, Park T. Effect of raw brown rice and Job's tear supplemented diet on serum and hepatic lipid concentrations, antioxidative system, and immune function of rats. J Korean Soc Food Sci Nutr. 2003; 32:197–206.25. Lee SG, Kim B, Yang Y, Pham TX, Park YK, Manatou J, Koo SI, Chun OK, Lee JY. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J Nutr Biochem. 2014; 25:404–411.
Article26. Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012; 2012:718617.
Article27. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003; 78:517S–520S.
Article28. Raigond B. Resistant starch in food: a review. J Sci Food Agric. 2015; 95:1968–1978.
Article29. Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002; 76:266S–273S.
Article30. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000; 342:836–843.
Article31. Bartucci M, Svensson S, Ricci-Vitiani L, Dattilo R, Biffoni M, Signore M, Ferla R, De Maria R, Surmacz E. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer. 2010; 17:823–833.
Article32. Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Möhlig M, Pfeiffer AF, Luft FC, Sharma AM. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003; 52:942–947.
Article33. Vowinkel T, Kalogeris TJ, Mori M, Krieglstein CF, Granger DN. Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Dig Dis Sci. 2004; 49:556–564.
Article34. Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996; 96:1027–1039.35. Park DK, Park HJ. Ethanol extract of Antrodia camphorata grown on germinated brown rice suppresses inflammatory responses in mice with acute DSS-induced colitis. Evid Based Complement Alternat Med. 2013; 2013:914524.36. Park MY, Kim JH, Park DS. Anti-inflammatory activities of hog millet (Panicum miliaceum L.) in murine macrophages through IRAK-4 signaling. Korean J Food Nutr. 2011; 24:268–272.
Article37. Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, Hong CP, Sinaiko AR. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009; 109:414–421.
Article38. Watanabe K, Kawamori T, Nakatsugi S, Wakabayashi K. COX-2 and iNOS, good targets for chemoprevention of colon cancer. Biofactors. 2000; 12:129–133.
Article39. Paixão J, Dinis TC, Almeida LM. Malvidin-3-glucoside protects endothelial cells up-regulating endothelial NO synthase and inhibiting peroxynitrite-induced NF-kB activation. Chem Biol Interact. 2012; 199:192–200.
Article40. Stephen AM, Cummings JH. Mechanism of action of dietary fibre in the human colon. Nature. 1980; 284:283–284.
Article41. Ferguson LR, Tasman-Jones C, Englyst H, Harris PJ. Comparative effects of three resistant starch preparations on transit time and short-chain fatty acid production in rats. Nutr Cancer. 2000; 36:230–237.
Article42. Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch - a review. Compr Rev Food Sci Food Saf. 2006; 5:1–17.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of mulberry twig extracts on dextran sulfate sodium-induced colitis mouse model
- Effects of Dextran Sulfate Sodium-Induced Ulcerative Colitis on the Disposition of Tofacitinib in Rats
- Histological Study of Experimental Colitis Induced by Dextran Sulfate Sodium
- Fine Structure of Goblet Cell Regeneration on Experimental Colitis Induced by Dextran Sulfate Sodium
- Inflammatory responses of C57BL/6NKorl mice to dextran sulfate sodium-induced colitis: comparison between three C57BL/6 N sub-strains