Allergy Asthma Immunol Res.
2017 Jan;9(1):61-69. 10.4168/aair.2017.9.1.61.
Inhibition of Allergic Response by Intranasal Selective NF-κB Decoy Oligodeoxynucleotides in a Murine Model of Allergic Rhinitis
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University College of Medicine, Seoul, Korea. dongkim@snu.ac.kr
- KMID: 2355895
- DOI: http://doi.org/10.4168/aair.2017.9.1.61
Abstract
- PURPOSE
It remains unknown whether local inhibition of Nuclear factor-kappa B (NF-κB) could have therapeutic value in the treatment of allergic rhinitis (AR). This study aimed to evaluate the effect of selective NF-κB inhibition using NF-κB decoy oligodeoxynucleotides (ODNs) for the local treatment of AR in ovalbumin (OVA)-sensitized wild-type mice.
METHODS
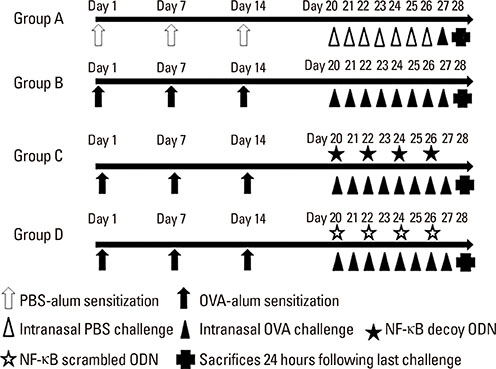
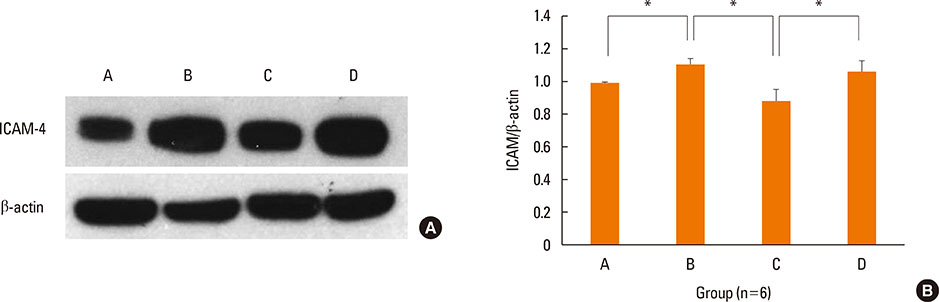
BALB/c mice were sensitized with OVA and alum, and then challenged intranasally with OVA. NF-κB decoy ODNs were given intranasally to the treatment group, and NF-κB scrambled ODNs were given to the sham treatment group. Allergic symptom scores, eosinophil infiltration, cytokine levels in the nasal mucosa, nasal lavage fluid, and spleen cell culture, serum total and OVA-specific immunoglobulins, as well as intercellular adhesion molecure-1 (ICAM-1) in the nasal mucosa, were analyzed.
RESULTS
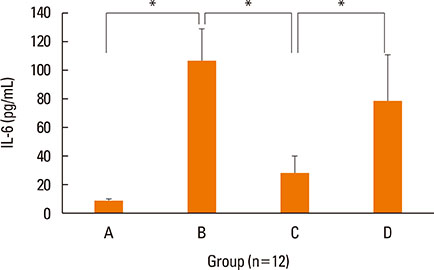
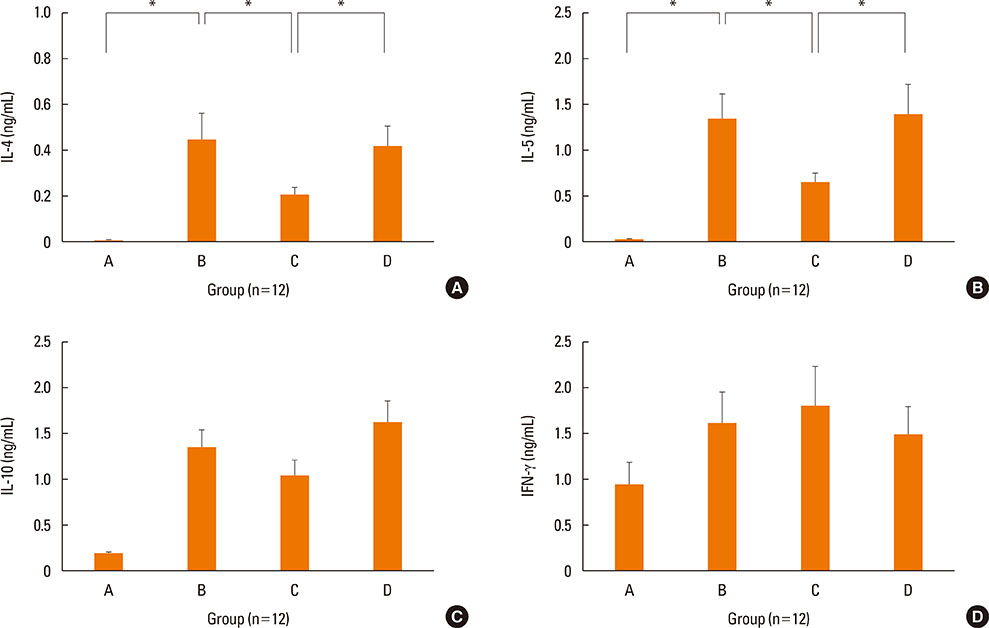
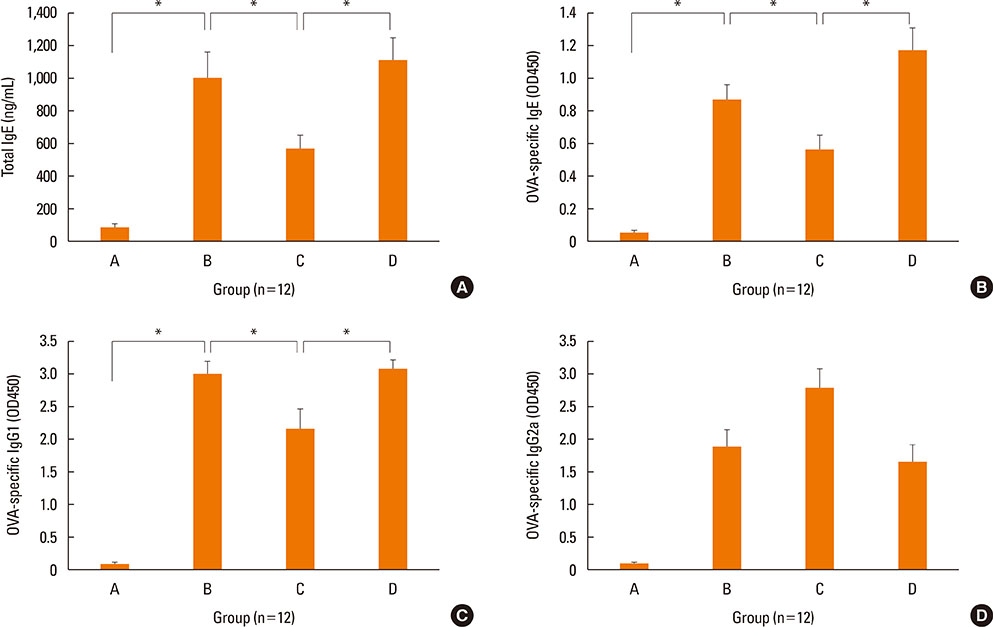
NF-κB decoy ODNs significantly reduced allergic symptoms and eosinophil infiltration in the nasal mucosa. They also suppressed serum levels of total IgE, OVA-specific IgE, and IgG1. IL-5 and TNF-α levels and the expression of ICAM-1 were decreased in the nasal mucosa of the treatment group compared to the positive control and sham treatment groups. In addition, IL-6 levels were significantly decreased in the nasal lavage fluid of the treatment group. Furthermore, NF-κB decoy ODNs significantly reduced expression of the systemic Th2 cytokines, IL-4 and IL-5 in spleen cell culture.
CONCLUSIONS
This study demonstrates for the first time that local NF-κB inhibition using NF-κB decoy ODNs suppressed the allergic response in a murine AR model. This shows the therapeutic potential of local NF-κB inhibition in the control of AR.
Keyword
MeSH Terms
-
Animals
Anti-Allergic Agents
Cell Culture Techniques
Cytokines
Eosinophils
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Intercellular Adhesion Molecule-1
Interleukin-4
Interleukin-5
Interleukin-6
Mice
Nasal Lavage Fluid
Nasal Mucosa
NF-kappa B
Oligodeoxyribonucleotides*
Ovalbumin
Ovum
Placebos
Rhinitis, Allergic*
Spleen
Anti-Allergic Agents
Cytokines
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Intercellular Adhesion Molecule-1
Interleukin-4
Interleukin-5
Interleukin-6
NF-kappa B
Oligodeoxyribonucleotides
Ovalbumin
Placebos
Figure
Reference
-
1. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998; 16:225–260.2. Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006; 13:738–747.3. Yamasaki K, Asai T, Shimizu M, Aoki M, Hashiya N, Sakonjo H, et al. Inhibition of NFkappaB activation using cis-element 'decoy' of NFkappaB binding site reduces neointimal formation in porcine balloon-injured coronary artery model. Gene Ther. 2003; 10:356–364.4. Crinelli R, Bianchi M, Gentilini L, Magnani M. Design and characterization of decoy oligonucleotides containing locked nucleic acids. Nucleic Acids Res. 2002; 30:2435–2443.5. Isomura I, Shintani Y, Yasuda Y, Tsujimura K, Morita A. Induction of regulatory dendritic cells by topical application of NF-kappaB decoy oligodeoxynucleotides. Immunol Lett. 2008; 119:49–56.6. Desmet C, Gosset P, Pajak B, Cataldo D, Bentires-Alj M, Lekeux P, et al. Selective blockade of NF-kappa B activity in airway immune cells inhibits the effector phase of experimental asthma. J Immunol. 2004; 173:5766–5775.7. Wang SZ, Ma FM, Zhao JD. Expressions of nuclear factor-kappa B p50 and p65 and their significance in the up-regulation of intercellular cell adhesion molecule-1 mRNA in the nasal mucosa of allergic rhinitis patients. Eur Arch Otorhinolaryngol. 2013; 270:1329–1334.8. Wang W, Zheng M. Nuclear factor kappa B pathway down-regulates aquaporin 5 in the nasal mucosa of rats with allergic rhinitis. Eur Arch Otorhinolaryngol. 2011; 268:73–81.9. Kim ST, Oh SC, Kim CW, Park C, Jang IH, Cha HE, et al. Expression of NF-κB and I-kB in allergic rhinitis. Korean J Otolaryngol-Head Neck Surg. 2000; 43:1191–1195.10. Percy DH, Barthold SW. Pathology of laboratory rodents and rabbits. 3rd ed. Ames (IA): Blackwell Publishing;2007.11. Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol. 2009; 37:249–255.12. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001; 107:7–11.13. Yamamoto Y, Gaynor RB. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci. 2004; 29:72–79.14. Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996; 14:649–683.15. Wang T, Li QH, Hao GP, Zhai J. Antitumor activity of decoy oligodeoxynucleotides targeted to NF-kappaB in vitro and in vivo. Asian Pac J Cancer Prev. 2010; 11:193–200.16. De Stefano D. Oligonucleotides decoy to NF-kappaB: becoming a reality? Discov Med. 2011; 12:97–105.17. Fang Y, Sun H, Zhai J, Zhang Y, Yi S, Hao G, et al. Antitumor activity of NF-kB decoy oligodeoxynucleotides in a prostate cancer cell line. Asian Pac J Cancer Prev. 2011; 12:2721–2726.18. Isomura I, Tsujimura K, Morita A. Antigen-specific peripheral tolerance induced by topical application of NF-kappaB decoy oligodeoxynucleotide. J Invest Dermatol. 2006; 126:97–104.19. Meltzer EO. The relationships of rhinitis and asthma. Allergy Asthma Proc. 2005; 26:336–340.20. Bao Z, Guan S, Cheng C, Wu S, Wong SH, Kemeny DM, et al. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am J Respir Crit Care Med. 2009; 179:657–665.21. Wang W, Zheng M. Mucin 5 subtype AC expression and upregulation in the nasal mucosa of allergic rhinitis rats. Otolaryngol Head Neck Surg. 2012; 147:1012–1019.22. Ueda A, Chandswang N, Ovary Z. The action of interleukin-4 on antigen-specific IgG1 and IgE production by interaction of in vivo primed B cells and carrier-specific cloned Th2 cells. Cell Immunol. 1990; 128:31–40.23. Lewkowich IP, Rempel JD, HayGlass KT. Antigen-specific versus total immunoglobulin synthesis: total IgE and IgG1, but not IgG2a levels predict murine antigen-specific responses. Int Arch Allergy Immunol. 2004; 133:145–153.24. Bossie A, Vitetta ES. IFN-gamma enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-gamma in class switching. Cell Immunol. 1991; 135:95–104.25. Fan D, Wang X, Wang M, Wang Y, Zhang L, Li Y, et al. Allergen-dependent differences in ILC2s frequencies in patients with allergic rhinitis. Allergy Asthma Immunol Res. 2016; 8:216–222.26. Mo JH, Kang EK, Quan SH, Rhee CS, Lee CH, Kim DY. Anti-tumor necrosis factor-alpha treatment reduces allergic responses in an allergic rhinitis mouse model. Allergy. 2011; 66:279–286.27. Yang CR, Hsieh SL, Ho FM, Lin WW. Decoy receptor 3 increases monocyte adhesion to endothelial cells via NF-kappa B-dependent up-regulation of intercellular adhesion molecule-1, VCAM-1, and IL-8 expression. J Immunol. 2005; 174:1647–1656.28. Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element "decoy" against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997; 3:894–899.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allergic Rhinitis Mouse Model
- Effect of Transcription Factor Decoy for NF-κB on the TNF-α Induced Cytokine and ICAM-1 Expression in Cultured HaCaT cells
- Inhibition of Allergic Response by CpG Motif Immunostimulatory Oligodeoxynucleotide in a Murine Model of Allergic Rhinitis
- Inhibition of Murine Allergic Response by Monoclonal Interleukin-4 Receptor Antibody
- House Dust Mite Allergic Rhinitis Model in C57BL/6 Mice