J Korean Neurosurg Soc.
2016 May;59(3):259-268. 10.3340/jkns.2016.59.3.259.
Sildenafil Ameliorates Advanced Glycation End Products-Induced Mitochondrial Dysfunction in HT-22 Hippocampal Neuronal Cells
- Affiliations
-
- 1Department of Neurosurgery, Pusan National University School of Medicine, Busan, Korea. gnsong@pusan.ac.kr
- 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Busan, Korea.
- 3Department of Physiology, Pusan National University School of Medicine, Busan, Korea.
- KMID: 2192103
- DOI: http://doi.org/10.3340/jkns.2016.59.3.259
Abstract
OBJECTIVE
Accumulation of advanced glycation end-products (AGE) and mitochondrial glycation is importantly implicated in the pathological changes of the brain associated with diabetic complications, Alzheimer disease, and aging. The present study was undertaken to determine whether sildenafil, a type 5 phosphodiesterase type (PDE-5) inhibitor, has beneficial effect on neuronal cells challenged with AGE-induced oxidative stress to preserve their mitochondrial functional integrity.
METHODS
HT-22 hippocampal neuronal cells were exposed to AGE and changes in the mitochondrial functional parameters were determined. Pretreatment of cells with sildenafil effectively ameliorated these AGE-induced deterioration of mitochondrial functional integrity.
RESULTS
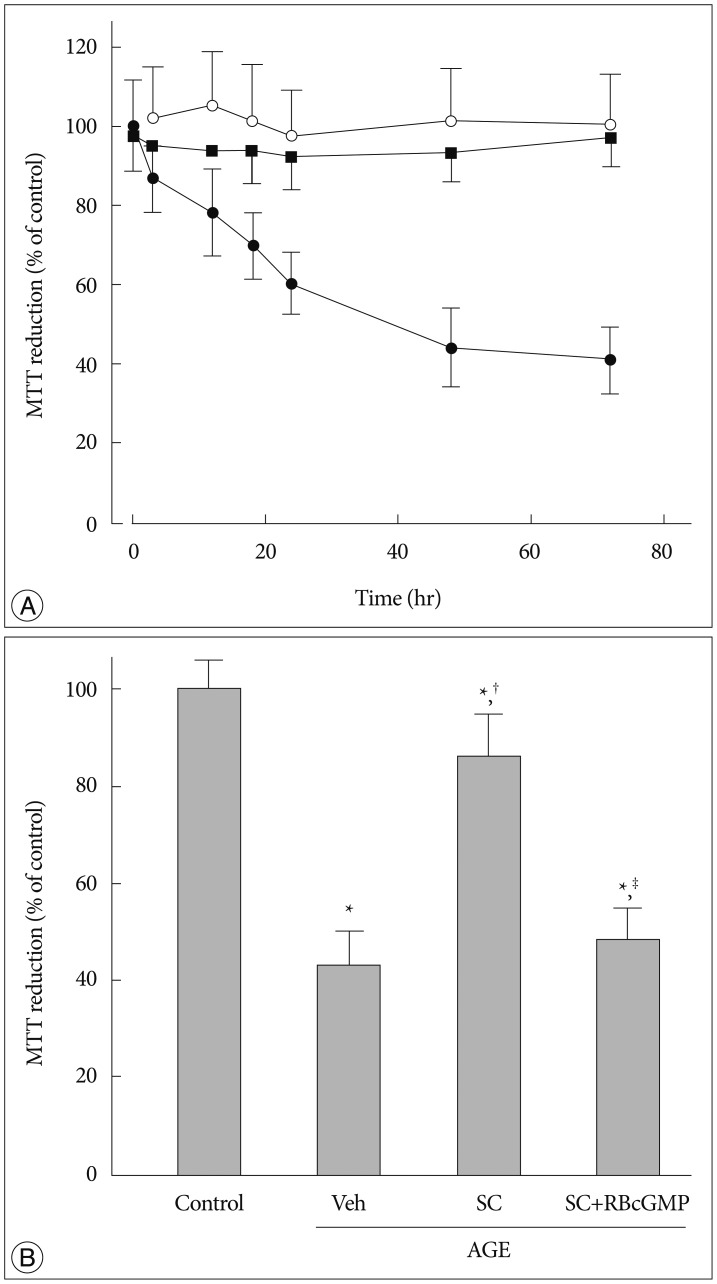
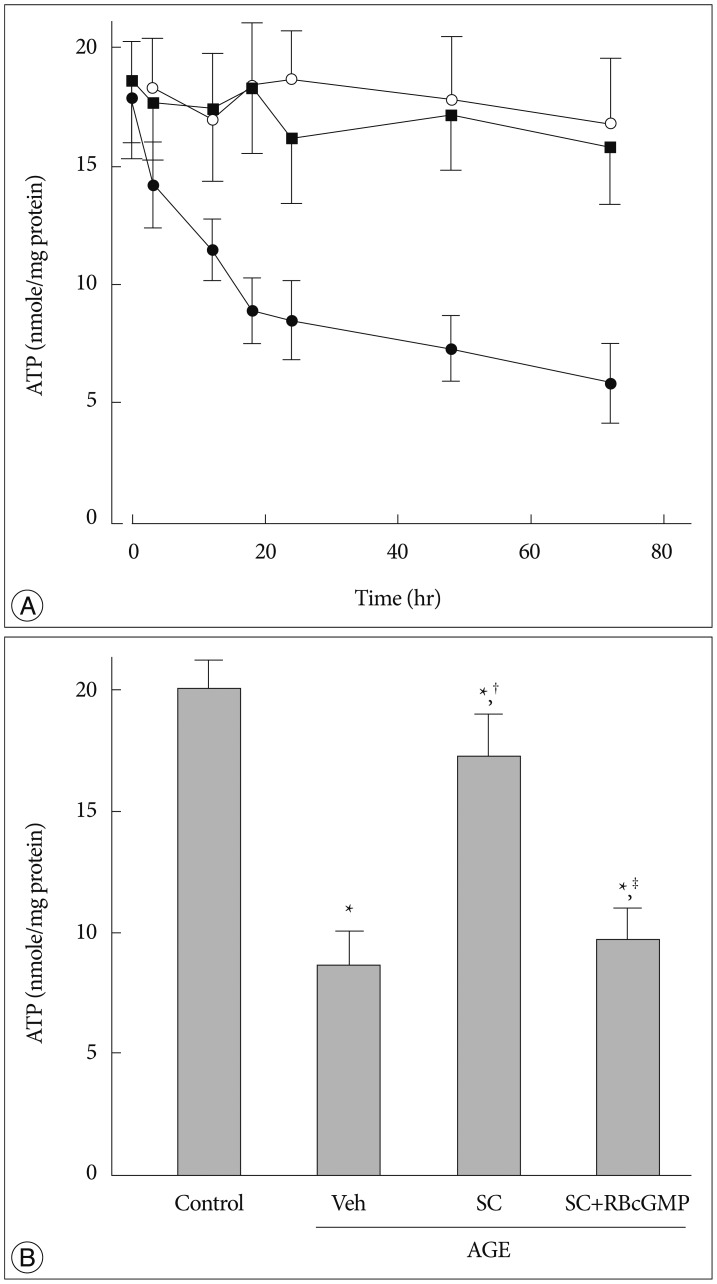
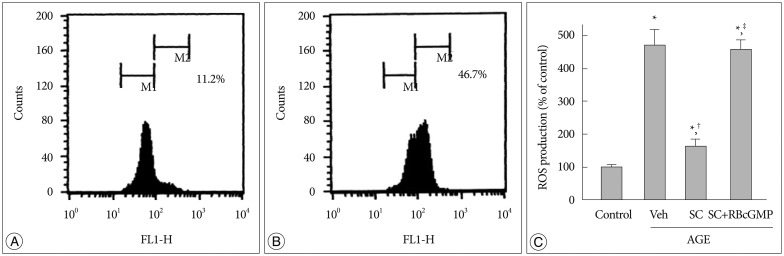
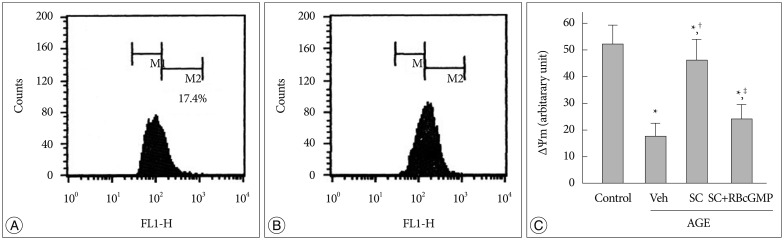
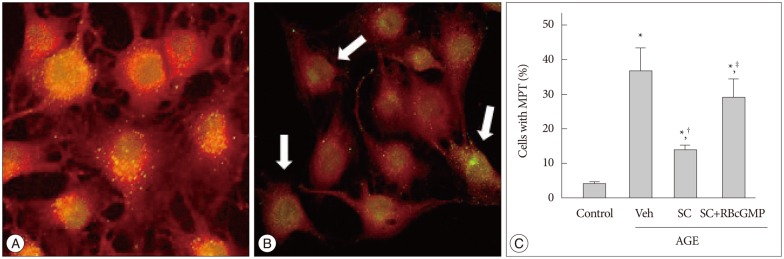
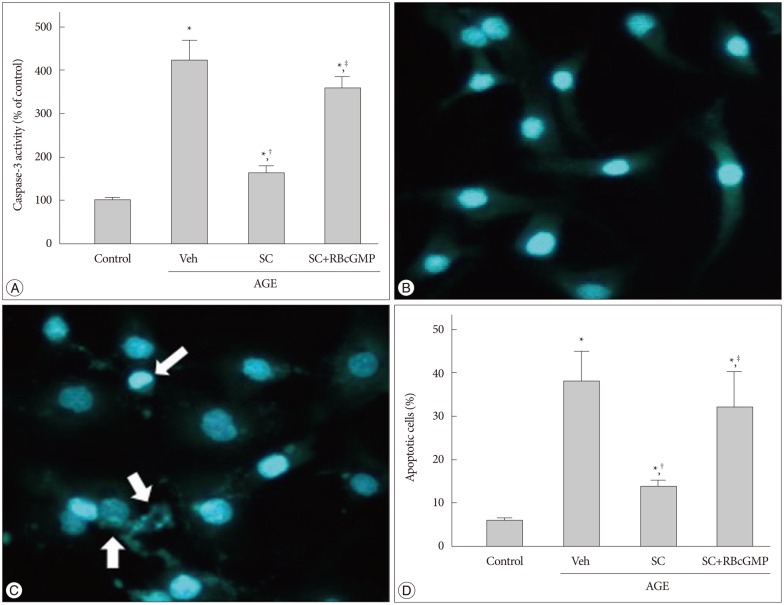
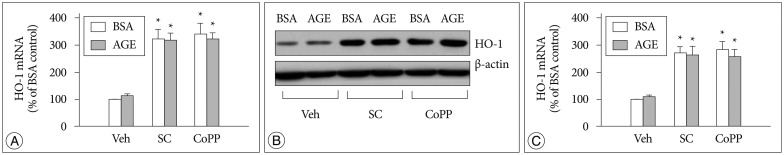
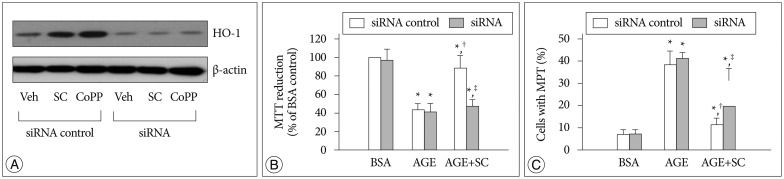
AGE-treated cells lost their mitochondrial functional integrity which was estimated by their MTT reduction ability and intracellular ATP concentration. These cells exhibited stimulated generation of reactive oxygen species (ROS), disruption of mitochondrial membrane potential, induction of mitochondrial permeability transition, and release of the cytochrome C, activation of the caspase-3 accompanied by apoptosis. Western blot analyses and qRT-PCR demonstrated that sildenafil increased the expression level of the heme oxygenase-1 (HO-1). CoPP and bilirubin, an inducer of HO-1 and a metabolic product of HO-1, respectively, provided a similar protective effects. On the contrary, the HO-1 inhibitor ZnPP IX blocked the effect of sildenafil. Transfection with HO-1 siRNA significantly reduced the protective effect of sildenafil on the loss of MTT reduction ability and MPT induction in AGE-treated cells.
CONCLUSION
Taken together, our results suggested that sildenafil provides beneficial effect to protect the HT-22 hippocampal neuronal cells against AGE-induced deterioration of mitochondrial integrity, and upregulation of HO-1 is involved in the underlying mechanism.
MeSH Terms
-
Adenosine Triphosphate
Aging
Alzheimer Disease
Apoptosis
Bilirubin
Blotting, Western
Brain
Caspase 3
Cytochromes c
Diabetes Complications
Glycosylation End Products, Advanced
Heme Oxygenase-1
Hippocampus
Membrane Potential, Mitochondrial
Mitochondria
Neurons*
Oxidative Stress
Permeability
Reactive Oxygen Species
RNA, Small Interfering
Transfection
Up-Regulation
Sildenafil Citrate
Adenosine Triphosphate
Bilirubin
Caspase 3
Cytochromes c
Glycosylation End Products, Advanced
Heme Oxygenase-1
RNA, Small Interfering
Reactive Oxygen Species
Figure
Reference
-
1. Abdel Aziz MT, El-Asmer MF, Mostafa T, Mostafa S, Atta H, Aziz Wassef MA, et al. Heme oxygenase vs. nitric oxide synthase in signaling mediating sildenafil citrate action. J Sex Med. 2007; 4(4 Pt 2):1098–1107. PMID: 17627722.
Article2. Aziz MT, Al-Asmar MF, Mostafa T, Atta H, Rashed L, Sabry D, et al. Assessment of heme oxygenase-1 (HO-1) activity in the cavernous tissues of sildenafil citrate-treated rats. Asian J Androl. 2007; 9:377–381. PMID: 17486279.
Article3. Aziz MT, Mostafa T, Atta H, Rashed L, Marzouk SA, Obaia EM, et al. The role of PDE5 inhibitors in heme oxygenase-cGMP relationship in rat cavernous tissues. J Sex Med. 2008; 5:1636–1645. PMID: 18208506.
Article4. Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta. 1998; 1366:211–223. PMID: 9714810.
Article5. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813–820. PMID: 11742414.
Article6. Burnett AL, Strong TD, Trock BJ, Jin L, Bivalacqua TJ, Musicki B. Serum biomarker measurements of endothelial function and oxidative stress after daily dosing of sildenafil in type 2 diabetic men with erectile dysfunction. J Urol. 2009; 181:245–251. PMID: 19013603.
Article7. Cadirci E, Halici Z, Odabasoglu F, Albayrak A, Karakus E, Unal D, et al. Sildenafil treatment attenuates lung and kidney injury due to overproduction of oxidant activity in a rat model of sepsis : a biochemical and histopathological study. Clin Exp Immunol. 2011; 166:374–384. PMID: 22059996.
Article8. Chen J. Heme oxygenase in neuroprotection : from mechanisms to therapeutic implications. Rev Neurosci. 2014; 25:269–280. PMID: 24501157.9. Choei H, Sasaki N, Takeuchi M, Yoshida T, Ukai W, Yamagishi S, et al. Glyceraldehyde-derived advanced glycation end products in Alzheimer's disease. Acta Neuropathol. 2004; 108:189–193. PMID: 15221334.
Article10. Choi DE, Jeong JY, Lim BJ, Chung S, Chang YK, Lee SJ, et al. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009; 297:F362–F370. PMID: 19474186.
Article11. Dar TA, Sheikh IA, Ganie SA, Ali R, Singh LR, Gan SH, et al. Molecular linkages between diabetes and Alzheimer's disease : current scenario and future prospects. CNS Neurol Disord Drug Targets. 2014; 13:290–298. PMID: 24059323.
Article12. Gaens KH, Stehouwer CD, Schalkwijk CG. Advanced glycation end-products and its receptor for advanced glycation endproducts in obesity. Curr Opin Lipidol. 2013; 24:4–11. PMID: 23298958.
Article13. Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil : from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006; 5:689–702. PMID: 16883306.
Article14. Hotston MR, Jeremy JY, Bloor J, Koupparis A, Persad R, Shukla N. Sildenafil inhibits the up-regulation of phosphodiesterase type 5 elicited with nicotine and tumour necrosis factor-alpha in cavernosal vascular smooth muscle cells : mediation by superoxide. BJU Int. 2007; 99:612–618. PMID: 17176295.
Article15. IDF Diabetes Atlas Group. Update of mortality attributable to diabetes for the IDF Diabetes Atlas : estimates for the year 2011. Diabetes Res Clin Pract. 2013; 100:277–279. PMID: 23506763.16. Jackson G, Gillies H, Osterloh I. Past, present, and future : a 7-year update of Viagra (sildenafil citrate). Int J Clin Pract. 2005; 59:680–691. PMID: 15924597.17. Jeremy JY, Koupparis A, Muzaffar S, Persad R, Angelini GD, Shukla N. Is the therapeutic action of sildenafil mediated partly through the inhibition of superoxide formation? BJU Int. 2005; 95:930–931. PMID: 15839904.
Article18. Kawahara G, Gasperini MJ, Myers JA, Widrick JJ, Eran A, Serafini PR, et al. Dystrophic muscle improvement in zebrafish via increased heme oxygenase signaling. Hum Mol Genet. 2014; 23:1869–1878. PMID: 24234649.
Article19. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008; 29:494–511. PMID: 18436709.
Article20. Konstantinopoulos A, Giannitsas K, Athanasopoulos A, Spathas D, Perimenis P. The impact of daily sildenafil on levels of soluble molecular markers of endothelial function in plasma in patients with erectile dysfunction. Expert Opin Pharmacother. 2009; 10:155–160. PMID: 19236190.
Article21. Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, et al. The mitochondrial permeability transition in cell death : a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998; 1366:177–196. PMID: 9714796.
Article22. Liu XM, Peyton KJ, Wang X, Durante W. Sildenafil stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells via distinct signaling pathways. Biochem Pharmacol. 2012; 84:1045–1054. PMID: 22864061.
Article23. Lyman GE, DeVincenzo JP. Determination of picogram amounts of ATP using the luciferin-luciferase enzyme system. Anal Biochem. 1967; 21:435–443. PMID: 6083397.
Article24. Marks GS. Heme oxygenase : the physiological role of one of its metabolites, carbon monoxide and interactions with zinc protoporphyrin, cobalt protoporphyrin and other metalloporphyrins. Cell Mol Biol (Noisy-le-grand). 1994; 40:863–870. PMID: 7849553.25. Mijnhout GS, Scheltens P, Diamant M, Biessels GJ, Wessels AM, Simsek S, et al. Diabetic encephalopathy : a concept in need of a definition. Diabetologia. 2006; 49:1447–1448. PMID: 16598451.
Article26. Morgan DM. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol. 1998; 79:179–183. PMID: 9463833.
Article27. Muzaffar S, Shukla N, Jeremy JY. Nicotinamide adenine dinucleotide phosphate oxidase : a promiscuous therapeutic target for cardiovascular drugs? Trends Cardiovasc Med. 2005; 15:278–282. PMID: 16297764.
Article28. Negi G, Kumar A, Kaundal RK, Gulati A, Sharma SS. Functional and biochemical evidence indicating beneficial effect of Melatonin and Nicotinamide alone and in combination in experimental diabetic neuropathy. Neuropharmacology. 2010; 58:585–592. PMID: 20005237.
Article29. Neuzil J, Stocker R. Bilirubin attenuates radical-mediated damage to serum albumin. FEBS Lett. 1993; 331:281–284. PMID: 8375511.
Article30. Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994; 269:16712–16719. PMID: 8206992.
Article31. Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets. 2008; 9:14–36. PMID: 18220710.
Article32. Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG. Crosstalk between advanced glycation and endoplasmic reticulum stress : emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab. 2012; 97:2231–2242. PMID: 22508704.
Article33. Pop-Busui R, Marinescu V, Van Huysen C, Li F, Sullivan K, Greene DA, et al. Dissection of metabolic, vascular, and nerve conduction interrelationships in experimental diabetic neuropathy by cyclooxygenase inhibition and acetyl-L-carnitine administration. Diabetes. 2002; 51:2619–2628. PMID: 12145179.
Article34. Pun PB, Murphy MP. Pathological significance of mitochondrial glycation. Int J Cell Biol. 2012; 2012:843505. PMID: 22778743.
Article35. Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, et al. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001; 15:2508–2514. PMID: 11689477.
Article36. Puzzo D, Loreto C, Giunta S, Musumeci G, Frasca G, Podda MV, et al. Effect of phosphodiesterase-5 inhibition on apoptosis and beta amyloid load in aged mice. Neurobiol Aging. 2014; 35:520–531. PMID: 24112792.
Article37. Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, et al. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer's disease mouse model. J Neurosci. 2009; 29:8075–8086. PMID: 19553447.
Article38. Rashid A. The efficacy and safety of PDE5 inhibitors. Clin Cornerstone. 2005; 7:47–56. PMID: 16156423.
Article39. Rolo AP, Palmeira CM. Diabetes and mitochondrial function : role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006; 212:167–178. PMID: 16490224.
Article40. Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy : futuristic strategies based on these targets. Int J Endocrinol. 2014; 2014:674987. PMID: 24883061.41. Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000; 1498:99–111. PMID: 11108954.
Article42. Shimizu F, Sano Y, Tominaga O, Maeda T, Abe MA, Kanda T. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-β by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol Aging. 2013; 34:1902–1912. PMID: 23428182.
Article43. Sima AA. Encephalopathies : the emerging diabetic complications. Acta Diabetol. 2010; 47:279–293. PMID: 20798963.
Article44. Takeuchi M, Yamagishi S. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer's disease. Curr Pharm Des. 2008; 14:973–978. PMID: 18473848.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Advanced Glycation End Products in Diabetic Vascular Complications
- Sildenafil Inhibits Advanced Glycation End Products-induced sFlt-1 Release Through Upregulation of Heme Oxygenase-1
- Spironolactone Attenuates Methylglyoxal-induced Cellular Dysfunction in MC3T3-E1 Osteoblastic Cells
- Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer's disease
- Control of Mitochondrial Dynamics by Fas-induced Caspase-8 Activation in Hippocampal Neurons