J Korean Med Sci.
2021 Oct;36(38):e265. 10.3346/jkms.2021.36.e265.
Spironolactone Attenuates Methylglyoxal-induced Cellular Dysfunction in MC3T3-E1 Osteoblastic Cells
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Kyung Hee University Hospital, Seoul, Korea
- 2Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul, Korea
- 3Department of Biomedical Laboratory Science, College of Health and Medical Sciences, Cheongju University, Cheongju, Korea
- KMID: 2520778
- DOI: http://doi.org/10.3346/jkms.2021.36.e265
Abstract
- Background
Methylglyoxal (MG) is associated with the pathogenesis of age- and diabetes-related complications. Spironolactone is a competitive antagonist of aldosterone that is widely employed in the treatment of hypertension and heart failure. This study examined the effects of spironolactone on MG-induced cellular dysfunction in MC3T3-E1 osteoblastic cells.
Methods
MC3T3-E1 cells were treated with spironolactone in the presence of MG. The mitochondrial function, bone formation activity, oxidative damage, inflammatory cytokines, glyoxalase I activity, and glutathione (GSH) were measured.
Results
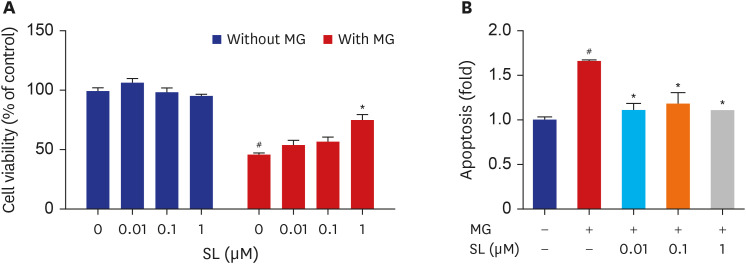
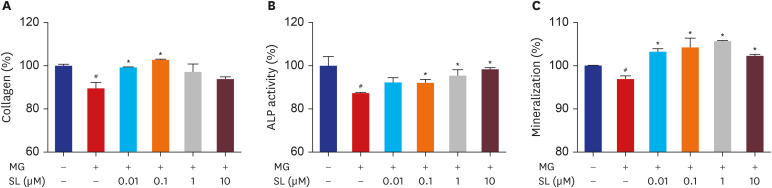
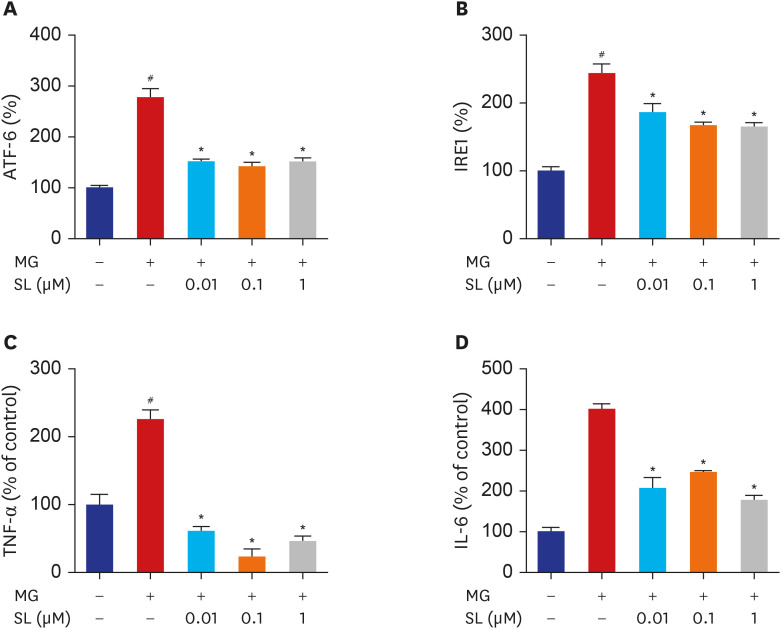
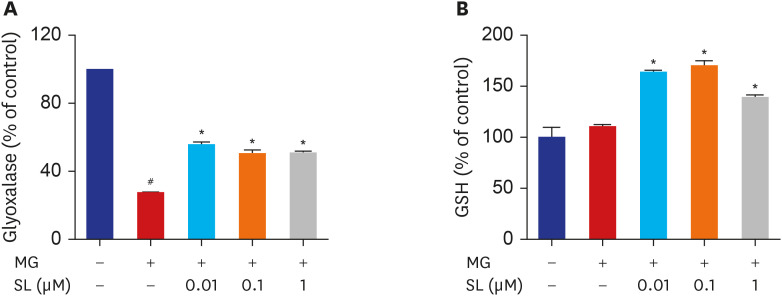
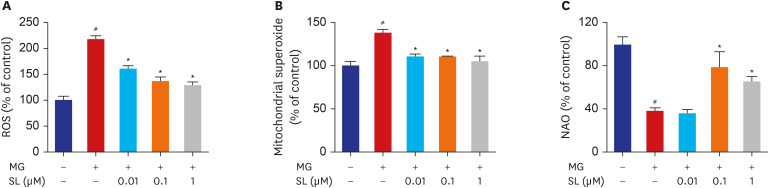
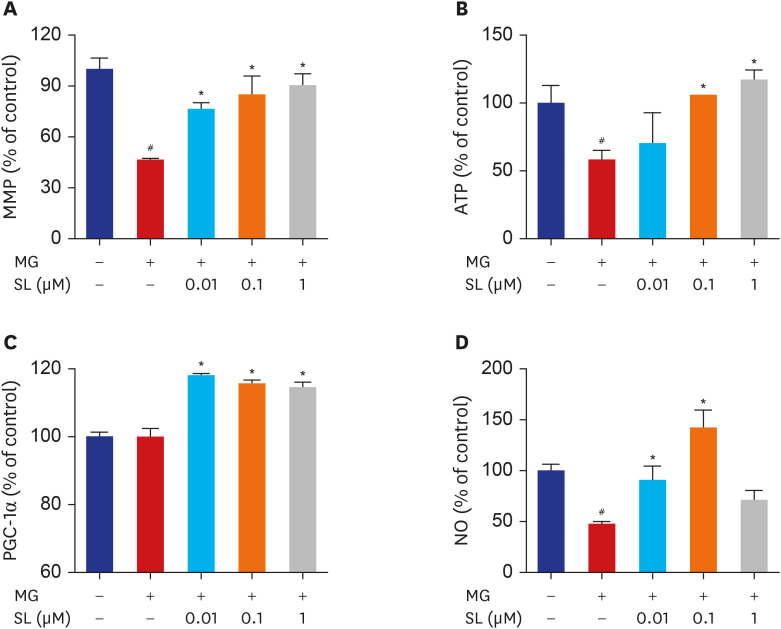
Pretreatment of MC3T3-E1 osteoblastic cells with spironolactone prevented MG-induced cell death, and improved bone formation activity. Spironolactone reduced MG-induced endoplasmic reticulum stress, production of intracellular reactive oxygen species, mitochondrial superoxides, cardiolipin peroxidation, and inflammatory cytokines. Pretreatment with spironolactone also increased the level of reduced GSH and the activity of glyoxalase I. MG induced mitochondrial dysfunction, but markers of mitochondrial biogenesis such as mitochondrial membrane potential, adenosine triphosphate, proliferator-activated receptor gamma coactivator 1α, and nitric oxide were significantly improved by treatment of spironolactone.
Conclusion
Spironolactone could prevent MG-induced cytotoxicity in MC3T3-E1 osteoblastic cells by reduction of oxidative stress. The oxidative stress reduction was explained by spironolactone's inhibition of advanced glycation end-product formation, restoring mitochondrial dysfunction, and anti-inflammatory effect.
Figure
Reference
-
1. Baik I. Projection of diabetes prevalence in Korean adults for the year 2030 using risk factors identified from national data. Diabetes Metab J. 2019; 43(1):90–96. PMID: 30398038.
Article2. Rosolová H, Petrlová B, Simon J, Sifalda P, Sípová I, Sefrna F. Macrovascular and microvascular complications in type 2 diabetes patients. Vnitr Lek. 2008; 54(3):229–237. PMID: 18522290.3. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007; 18(4):427–444. PMID: 17068657.
Article4. Kanazawa I, Sugimoto T. Diabetes mellitus-induced bone fragility. Intern Med. 2018; 57(19):2773–2785. PMID: 29780142.
Article5. Rabbani N, Thornalley PJ. Dicarbonyls linked to damage in the powerhouse: glycation of mitochondrial proteins and oxidative stress. Biochem Soc Trans. 2008; 36(Pt 5):1045–1050. PMID: 18793186.
Article6. Miyazawa T, Nakagawa K, Shimasaki S, Nagai R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012; 42(4):1163–1170. PMID: 20957396.
Article7. Angeloni C, Zambonin L, Hrelia S. Role of methylglyoxal in Alzheimer's disease. BioMed Res Int. 2014; 2014:238485. PMID: 24734229.
Article8. Turkseven S, Ertuna E, Yetik-Anacak G, Yasa M. Methylglyoxal causes endothelial dysfunction: the role of endothelial nitric oxide synthase and AMP-activated protein kinase α. J Basic Clin Physiol Pharmacol. 2014; 25(1):109–115. PMID: 24127540.
Article9. Chan WH, Wu HJ, Shiao NH. Apoptotic signaling in methylglyoxal-treated human osteoblasts involves oxidative stress, c-Jun N-terminal kinase, caspase-3, and p21-activated kinase 2. J Cell Biochem. 2007; 100(4):1056–1069. PMID: 17131386.
Article10. Petramala L, Zinnamosca L, Settevendemmie A, Marinelli C, Nardi M, Concistrè A, et al. Bone and mineral metabolism in patients with primary aldosteronism. Int J Endocrinol. 2014; 2014:836529. PMID: 24864141.
Article11. Ceccoli L, Ronconi V, Giovannini L, Marcheggiani M, Turchi F, Boscaro M, et al. Bone health and aldosterone excess. Osteoporos Int. 2013; 24(11):2801–2807. PMID: 23695421.
Article12. Shi S, Lu C, Tian H, Ren Y, Chen T. Primary aldosteronism and bone metabolism: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020; 11:574151. PMID: 33101208.
Article13. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999; 341(10):709–717. PMID: 10471456.14. Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, et al. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation. 2005; 111(7):871–878. PMID: 15710759.
Article15. Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, et al. Loss of bone minerals and strength in rats with aldosteronism. Am J Physiol Heart Circ Physiol. 2004; 287(5):H2023–H2026. PMID: 15475529.
Article16. Adolf C, Braun LT, Fuss CT, Hahner S, Künzel H, Handgriff L, et al. Spironolactone reduces biochemical markers of bone turnover in postmenopausal women with primary aldosteronism. Endocrine. 2020; 69(3):625–633. PMID: 32594379.
Article17. Carbone LD, Cross JD, Raza SH, Bush AJ, Sepanski RJ, Dhawan S, et al. Fracture risk in men with congestive heart failure risk reduction with spironolactone. J Am Coll Cardiol. 2008; 52(2):135–138. PMID: 18598893.18. Beavan S, Horner A, Bord S, Ireland D, Compston J. Colocalization of glucocorticoid and mineralocorticoid receptors in human bone. J Bone Miner Res. 2001; 16(8):1496–1504. PMID: 11499872.
Article19. Liu W, Gong W, He M, Liu Y, Yang Y, Wang M, et al. Spironolactone protects against diabetic cardiomyopathy in streptozotocin-induced diabetic rats. J Diabetes Res. 2018; 2018:9232065. PMID: 30406151.
Article20. Mayyas F, Alzoubi KH, Bonyan R. The role of spironolactone on myocardial oxidative stress in rat model of streptozotocin-induced diabetes. Cardiovasc Ther. 2017; 35(2):e12242.
Article21. Suh KS, Chon S, Choi EM. Bergenin increases osteogenic differentiation and prevents methylglyoxal-induced cytotoxicity in MC3T3-E1 osteoblasts. Cytotechnology. 2018; 70(1):215–224. PMID: 28895006.
Article22. Suh KS, Chon S, Choi EM. Protective effects of piceatannol on methylglyoxal-induced cytotoxicity in MC3T3-E1 osteoblastic cells. Free Radic Res. 2018; 52(6):712–723. PMID: 29792365.
Article23. Schroeder P, Pohl C, Calles C, Marks C, Wild S, Krutmann J. Cellular response to infrared radiation involves retrograde mitochondrial signaling. Free Radic Biol Med. 2007; 43(1):128–135. PMID: 17561101.
Article24. Wang H, Meng QH, Chang T, Wu L. Fructose-induced peroxynitrite production is mediated by methylglyoxal in vascular smooth muscle cells. Life Sci. 2006; 79(26):2448–2454. PMID: 16950408.
Article25. Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010; 140(6):900–917. PMID: 20303879.
Article26. Forbes JM, Cooper ME, Thallas V, Burns WC, Thomas MC, Brammar GC, et al. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes. 2002; 51(11):3274–3282. PMID: 12401719.
Article27. Santel T, Pflug G, Hemdan NY, Schäfer A, Hollenbach M, Buchold M, et al. Curcumin inhibits glyoxalase 1: a possible link to its anti-inflammatory and anti-tumor activity. PLoS One. 2008; 3(10):e3508. PMID: 18946510.28. Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, et al. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998; 101(5):1142–1147. PMID: 9486985.
Article29. Miyata T, van Ypersele de Strihou C, Imasawa T, Yoshino A, Ueda Y, Ogura H, et al. Glyoxalase I deficiency is associated with an unusual level of advanced glycation end products in a hemodialysis patient. Kidney Int. 2001; 60(6):2351–2359. PMID: 11737610.
Article30. Thornalley PJ. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003; 31(Pt 6):1343–1348. PMID: 14641060.31. Abordo EA, Minhas HS, Thornalley PJ. Accumulation of alpha-oxoaldehydes during oxidative stress: a role in cytotoxicity. Biochem Pharmacol. 1999; 58(4):641–648. PMID: 10413301.32. Kiefel BR, Gilson PR, Beech PL. Cell biology of mitochondrial dynamics. Int Rev Cytol. 2006; 254:151–213. PMID: 17147999.
Article33. Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB, et al. Mitochondrial calpain-1 disrupts ATP synthase and induces superoxide generation in type 1 diabetic hearts: a novel mechanism contributing to diabetic cardiomyopathy. Diabetes. 2016; 65(1):255–268. PMID: 26470784.
Article34. Matafome P, Sena C, Seiça R. Methylglyoxal, obesity, and diabetes. Endocrine. 2013; 43(3):472–484. PMID: 22983866.
Article35. Berlanga J, Cibrian D, Guillén I, Freyre F, Alba JS, Lopez-Saura P, et al. Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin Sci (Lond). 2005; 109(1):83–95. PMID: 15755259.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lactoferrin Constitutively Enhances Differentiation of Osteoblastic MC3T3-E1 Cells in Vitro
- Insulin growth factor binding protein-3 enhances dental implant osseointegration against methylglyoxal-induced bone deterioration in a rat model
- Bone Morphogenetic Protein-2 Desensitizes MC3T3-E1 Osteoblastic Cells to Estrogen Through Transcriptional Downregulation of Estrogen Receptor 1
- Effect of Centrifugal Force on Cellular Activity of Osteoblastic MC3T3-E1 Cells in vitro
- The Role of Nuclear Factor-E2-Related Factor 1 in the Oxidative Stress Response in MC3T3-E1 Osteoblastic Cells