J Menopausal Med.
2014 Aug;20(2):57-68. 10.6118/jmm.2014.20.2.57.
Sildenafil Inhibits Advanced Glycation End Products-induced sFlt-1 Release Through Upregulation of Heme Oxygenase-1
- Affiliations
-
- 1Department Obstetrics and Gynecology, Pusan National University, School of Medicine, Yangsan, Korea. ohchoi@pusan.ac.kr
- KMID: 2325443
- DOI: http://doi.org/10.6118/jmm.2014.20.2.57
Abstract
OBJECTIVES
We examined the effect of sildenafil citrate on advanced glycation end products (AGEs)-induced soluble fms-like tyrosine kinase 1 (sFlt-1) release in JEG-3 choriocarcinoma cells.
METHODS
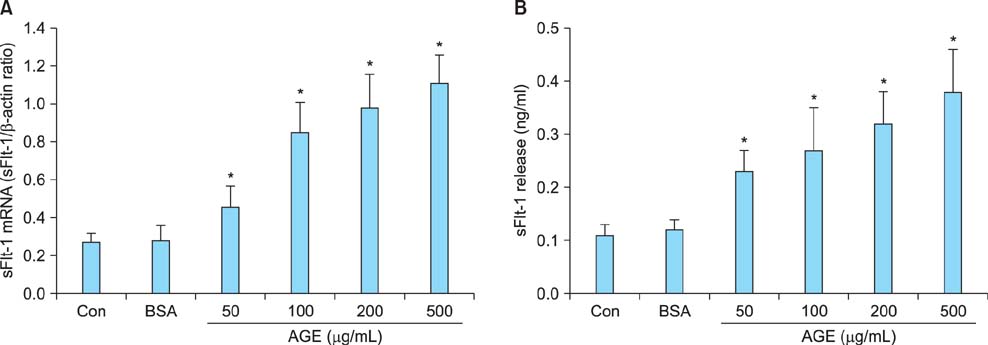
Cells were incubated with control bovine serum albumin (BSA) or AGEs-BSA, and expression of sFlt-1 mRNA and protein release was determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. AGEs-BSA increased sFlt-1 mRNA expression and protein release in a dose-dependent manner.
RESULTS
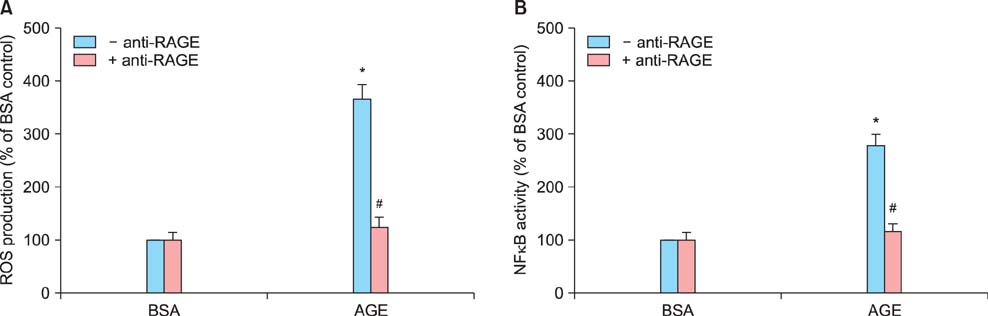
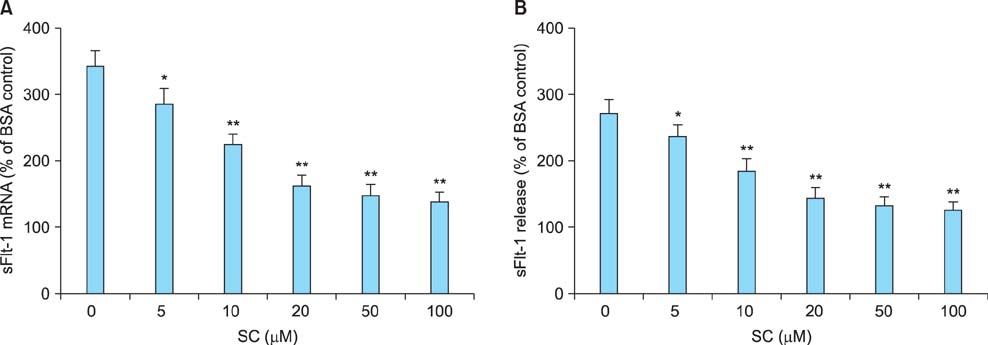
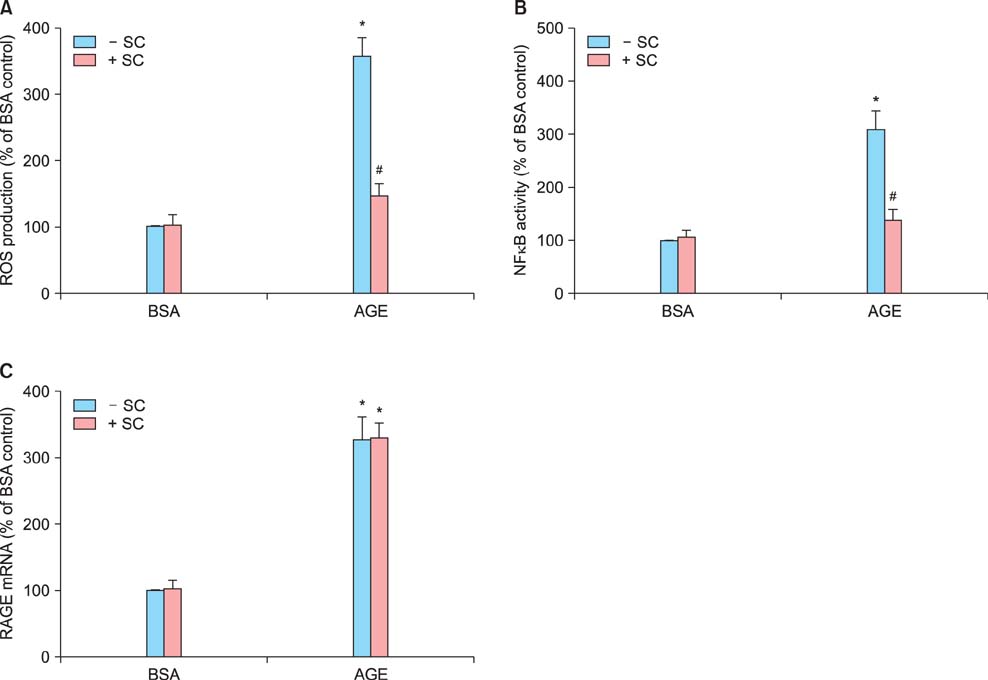
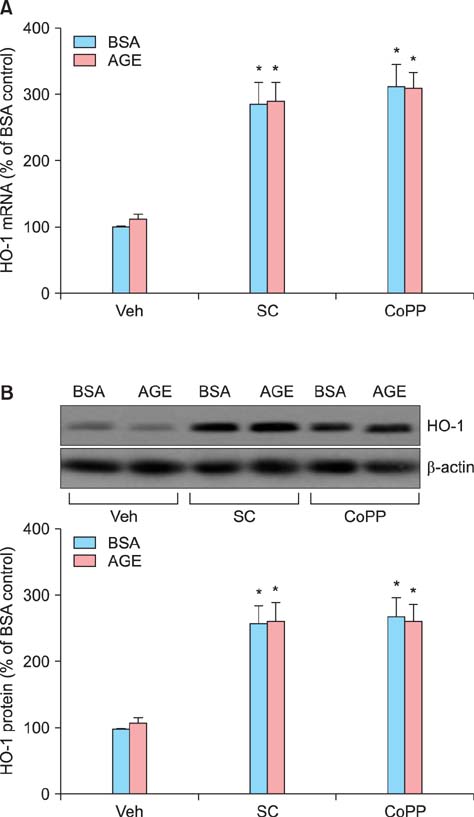
Sildenafil citrate suppressed sFlt-1 mRNA expression and protein release in cells treated with AGEs-BSA in a dose-dependent manner. Likewise, it inhibited the increase of reactive oxygen species (ROS) production and NF-kappaB activity in these cells. Cobalt protoporphyrin (CoPP) and bilirubin also inhibited sFlt-1 release and ROS production in cells treated with AGEs-BSA, whereas zinc protoporphyrin IX (ZnPP IX) antagonized the effect of sildenafil citrate. In cells transfected with the heme oxygenase-1 (HO-1) siRNA, sildenafil citrate failed to inhibit the sFlt-1 release and ROS production.
CONCLUSION
These results strongly suggest that sildenafil citrate inhibits sFlt-1 release and ROS production in cells treated with AGEs-BSA through upregulation of the HO-1 expression in JEG-3 cells.
MeSH Terms
-
Bilirubin
Choriocarcinoma
Citric Acid
Cobalt
Enzyme-Linked Immunosorbent Assay
Female
Glycosylation End Products, Advanced
Heme Oxygenase-1*
NF-kappa B
Pregnancy
Reactive Oxygen Species
RNA, Messenger
RNA, Small Interfering
Serum Albumin, Bovine
Up-Regulation*
Vascular Endothelial Growth Factor Receptor-1
Zinc
Sildenafil Citrate
Bilirubin
Citric Acid
Cobalt
Glycosylation End Products, Advanced
Heme Oxygenase-1
NF-kappa B
RNA, Messenger
RNA, Small Interfering
Reactive Oxygen Species
Serum Albumin, Bovine
Vascular Endothelial Growth Factor Receptor-1
Zinc
Figure
Reference
-
1. Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003; 41:437–445.2. Turner JA. Diagnosis and management of pre-eclampsia: an update. Int J Womens Health. 2010; 2:327–337.3. Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010; 14:528–552.4. Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, Halmesmaki E. Amniotic fluid--soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol. 2000; 95:353–357.5. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111:649–658.6. Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004; 95:884–891.7. Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010; 14:1857–1867.8. Leaños-Miranda A, Campos-Galicia I, Isordia-Salas I, Rivera-Leaños R, Romero-Arauz JF, Ayala-Méndez JA, et al. Changes in circulating concentrations of soluble fms-like tyrosine kinase-1 and placental growth factor measured by automated electrochemiluminescence immunoassays methods are predictors of preeclampsia. J Hypertens. 2012; 30:2173–2181.9. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813–820.10. Vlassara H, Striker GE. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol. 2011; 7:526–539.11. Gaens KH, Stehouwer CD, Schalkwijk CG. Advanced glycation endproducts and its receptor for advanced glycation endproducts in obesity. Curr Opin Lipidol. 2013; 24:4–11.12. Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab. 2012; 97:2231–2242.13. Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou SA, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Hum Reprod Update. 2011; 17:741–760.14. Chekir C, Nakatsuka M, Noguchi S, Konishi H, Kamada Y, Sasaki A, et al. Accumulation of advanced glycation end products in women with preeclampsia: possible involvement of placental oxidative and nitrative stress. Placenta. 2006; 27:225–233.15. Oliver EA, Buhimschi CS, Dulay AT, Baumbusch MA, Abdel-Razeq SS, Lee SY, et al. Activation of the receptor for advanced glycation end products system in women with severe preeclampsia. J Clin Endocrinol Metab. 2011; 96:689–698.16. Guedes-Martins L, Matos L, Soares A, Silva E, Almeida H. AGEs, contributors to placental bed vascular changes leading to preeclampsia. Free Radic Res. 2013; 47:Suppl 1. 70–80.17. Huang QT, Zhang M, Zhong M, Yu YH, Liang WZ, Hang LL, et al. Advanced glycation end products as an upstream molecule triggers ROS-induced sFlt-1 production in extravillous trophoblasts: a novel bridge between oxidative stress and preeclampsia. Placenta. 2013; 34:1177–1182.18. Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006; 5:689–702.19. Downing J. Sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2010; 29:248–250. author reply 51-2.20. Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009; 28:369–382.21. Kim TH, Lee HH, Kwak JJ. Conservative management of abnormally invasive placenta: choriocarcinoma with uterine arteriovenous fistula from remnant invasive placenta. Acta Obstet Gynecol Scand. 2013; 92:989–990.22. Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate improves fetal outcomes in pregnant, L-NAME treated, Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol. 2010; 149:22–26.23. George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2013; 305:R397–R403.24. Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol. 2011; 157:136–140.25. Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, et al. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta. 2011; 32:Suppl. S49–S54.26. Mun MJ, Kim JH, Kim TH, Hwang JY, Jang WC. Associations between estrogen receptor gene polymorphisms and endometriosis. J Korean Soc Menopause. 2013; 19:64–73.27. Groesch KA, Torry RJ, Wilber AC, Abrams R, Bieniarz A, Guilbert LJ, et al. Nitric oxide generation affects pro- and anti-angiogenic growth factor expression in primary human trophoblast. Placenta. 2011; 32:926–931.28. Franke S, Siggelkow H, Wolf G, Hein G. Advanced glycation endproducts influence the mRNA expression of RAGE, RANKL and various osteoblastic genes in human osteoblasts. Arch Physiol Biochem. 2007; 113:154–161.29. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010; 107:1058–1070.30. Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012; 1820:663–671.31. Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007; 115:1789–1797.32. George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011; 57:941–948.33. George EM, Colson D, Dixon J, Palei AC, Granger JP. Heme oxygenase-1 attenuates hypoxia-induced sFlt-1 and oxidative stress in placental villi through its metabolic products CO and bilirubin. Int J Hypertens. 2012; 2012:486053.34. Marks GS. Heme oxygenase: the physiological role of one of its metabolites, carbon monoxide and interactions with zinc protoporphyrin, cobalt protoporphyrin and other metalloporphyrins. Cell Mol Biol (Noisy-le-grand). 1994; 40:863–870.35. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007; 335:974.36. Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006; 47:502–508.37. Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000; 1498:99–111.38. Herraiz S, Pellicer B, Serra V, Cauli O, Cortijo J, Felipo V, et al. Sildenafil citrate improves perinatal outcome in fetuses from pre-eclamptic rats. BJOG. 2012; 119:1394–1402.39. Stanley JL, Andersson IJ, Poudel R, Rueda-Clausen CF, Sibley CP, Davidge ST, et al. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension. 2012; 59:1021–1028.40. Abram SR, Alexander BT, Bennett WA, Granger JP. Role of neuronal nitric oxide synthase in mediating renal hemodynamic changes during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001; 281:R1390–R1393.41. Anumba DO, Robson SC, Boys RJ, Ford GA. Nitric oxide activity in the peripheral vasculature during normotensive and preeclamptic pregnancy. Am J Physiol. 1999; 277:H848–H854.42. Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, et al. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993; 7:566–571.43. Cao J, Inoue K, Li X, Drummond G, Abraham NG. Physiological significance of heme oxygenase in hypertension. Int J Biochem Cell Biol. 2009; 41:1025–1033.44. Botros FT, Schwartzman ML, Stier CT Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005; 68:2745–2755.45. Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, et al. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001; 38:210–215.46. Yang L, Quan S, Nasjletti A, Laniado-Schwartzman M, Abraham NG. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension. 2004; 43:1221–1226.47. Neuzil J, Stocker R. Bilirubin attenuates radical-mediated damage to serum albumin. FEBS Lett. 1993; 331:281–284.48. Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994; 269:16712–16719.49. Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005; 57:585–630.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sildenafil Ameliorates Advanced Glycation End Products-Induced Mitochondrial Dysfunction in HT-22 Hippocampal Neuronal Cells
- Mitochondrial Channel Opener Diazoxide Attenuates Hypoxia-Induced sFlt-1 Release in Human Choriocarcinoma Cells
- Correlation of advanced glycation end products and heme oxygenase-1 in Korean diabetic patients
- The Role of Advanced Glycation End Products in Diabetic Vascular Complications
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases