Anat Cell Biol.
2017 Jun;50(2):124-134. 10.5115/acb.2017.50.2.124.
Baicalein, wogonin, and Scutellaria baicalensis ethanol extract alleviate ovalbumin-induced allergic airway inflammation and mast cell-mediated anaphylactic shock by regulation of Th1/Th2 imbalance and histamine release
- Affiliations
-
- 1Department of Anatomy, Chonbuk National University Medical School, Jeonju, Korea. okchai1004@jbnu.ac.kr
- 2Institute for Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea.
- 3Department of Anatomy, College of Korean Medicine, Woosuk University, Samrye, Korea.
- 4Food Biotechnology Program, Korea University of Science and Technology, Daejeon, Korea.
- 5Division of Nutrition and Metabolism Research, Korea Food Research Institute, Seongnam, Korea.
- KMID: 2451238
- DOI: http://doi.org/10.5115/acb.2017.50.2.124
Abstract
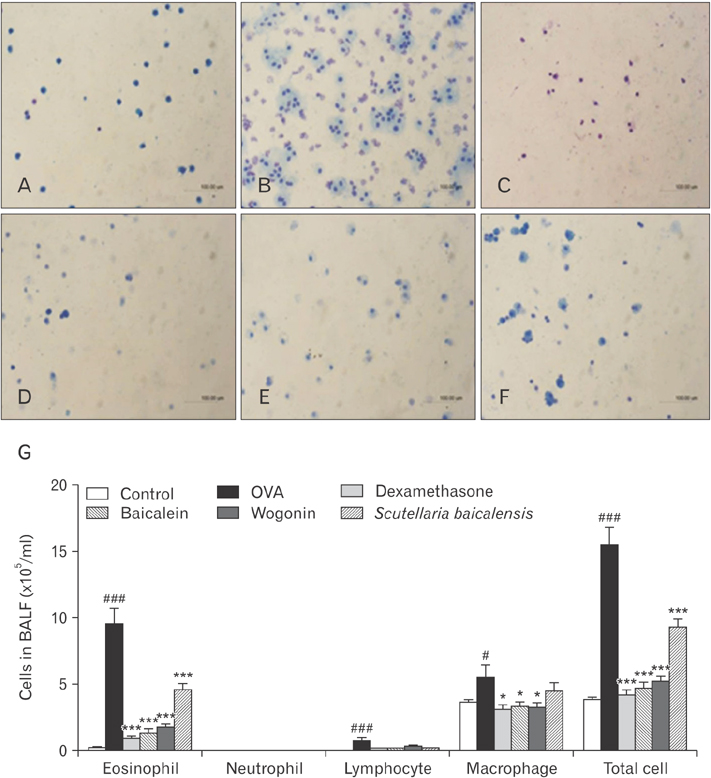
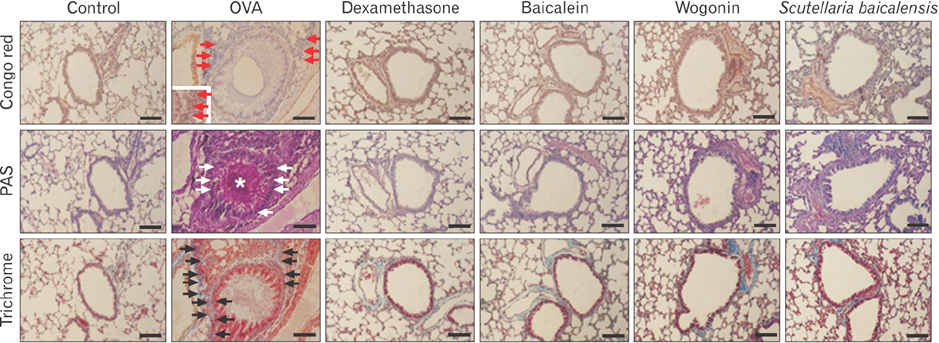
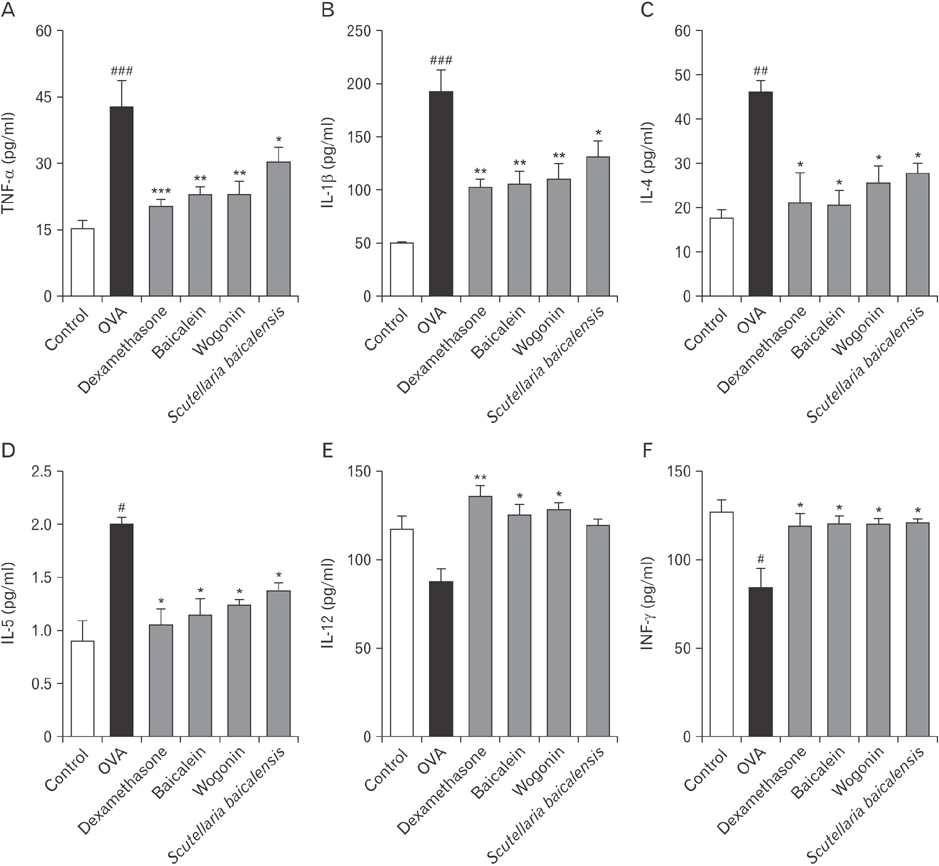
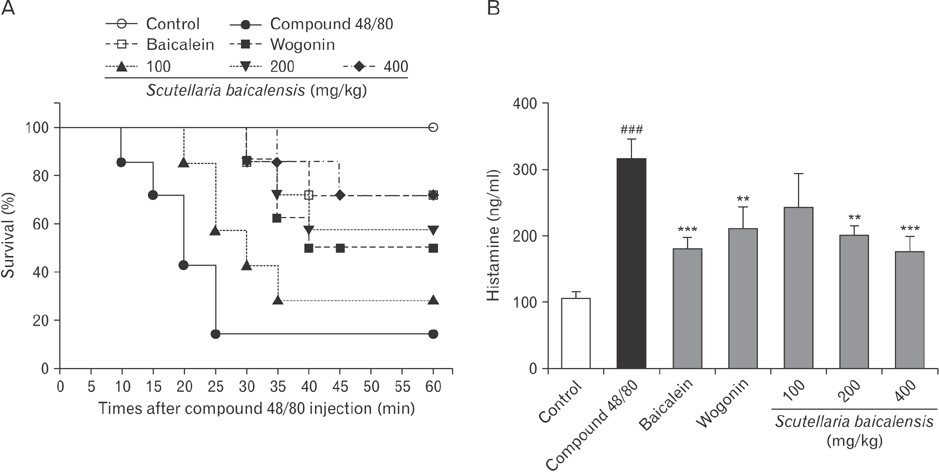
- Asthma is characterized by chronic inflammation, goblet cell hyperplasia, the aberrant production of the Th2 cytokines, and eosinophil infiltration into the lungs. In this study, we examined the effects of baicalein, wogonin, and Scutellaria baicalensis ethanol extract on ovalbumin (OVA)-induced asthma by evaluating Th1/Th2 cytokine levels, histopathologic analysis, and compound 48/80-induced systemic anaphylaxis and mast cell activation, focusing on the histamine release from rat peritoneal mast cells. Baicalein, wogonin, and S. baicalensis ethanol extract also decreased the number of inflammatory cells especially eosinophils and downregulated peribronchial and perivascular inflammation in the lungs of mice challenged by OVA. Baicalein, wogonin, and S. baicalensis ethanol extract significantly reduced the levels of tumor necrosis factor α, interleukin (IL)-1β, IL-4, IL-5 and the production of OVA-specific IgE and IgG1, and upregulated the level of interferon-γ and OVA-specific IgG2a. In addition, oral administration of baicalein, wogonin, and S. baicalensis ethanol extract inhibited compound 48/80-induced systemic anaphylaxis and plasma histamine release in mice. Moreover, baicalein, wogonin, and S. baicalensis ethanol extract suppressed compound 48/80-induced mast cell degranulation and histamine release from rat peritoneal mast cells. Conclusively, baicalein and wogonin as major flavonoids of S. baicalensis may have therapeutic potential for allergic asthma through modulation of Th1/Th2 cytokine imbalance and histamine release from mast cells.
Keyword
MeSH Terms
-
Administration, Oral
Anaphylaxis*
Animals
Asthma
Cytokines
Eosinophils
Ethanol*
Flavonoids
Goblet Cells
Histamine Release*
Histamine*
Hyperplasia
Immunoglobulin E
Immunoglobulin G
Inflammation*
Interleukin-4
Interleukin-5
Interleukins
Lung
Mast Cells
Mice
Ovalbumin
Ovum
Plasma
Rats
Scutellaria baicalensis*
Scutellaria*
Tumor Necrosis Factor-alpha
Cytokines
Ethanol
Flavonoids
Histamine
Immunoglobulin E
Immunoglobulin G
Interleukin-4
Interleukin-5
Interleukins
Ovalbumin
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy. 2014; 7:53–65.2. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015; 16:45–56.3. Kim YS, Choi SJ, Choi JP, Jeon SG, Oh S, Lee BJ, Gho YS, Lee CG, Zhu Z, Elias JA, Kim YK. IL-12-STAT4-IFN-gamma axis is a key downstream pathway in the development of IL-13-mediated asthma phenotypes in a Th2 type asthma model. Exp Mol Med. 2010; 42:533–546.4. Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA, Bradding P. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015; 7:301ra129.5. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009; 180:388–395.6. Troy NM, Hollams EM, Holt PG, Bosco A. Differential gene network analysis for the identification of asthma-associated therapeutic targets in allergen-specific T-helper memory responses. BMC Med Genomics. 2016; 9:9.7. Punnonen J, Aversa G, Cocks BG, de Vries JE. Role of interleukin-4 and interleukin-13 in synthesis of IgE and expression of CD23 by human B cells. Allergy. 1994; 49:576–586.8. Abdelmotelb AM, Rose-Zerilli MJ, Barton SJ, Holgate ST, Walls AF, Holloway JW. Alpha-tryptase gene variation is associated with levels of circulating IgE and lung function in asthma. Clin Exp Allergy. 2014; 44:822–830.9. Cardamone C, Parente R, Feo GD, Triggiani M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol Lett. 2016; 178:10–14.10. Dahlin JS, Hallgren J. Mast cell progenitors: origin, development and migration to tissues. Mol Immunol. 2015; 63:9–17.11. Galli SJ. The mast cell-IgE paradox: from homeostasis to anaphylaxis. Am J Pathol. 2016; 186:212–224.12. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014; 62:698–738.13. Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, Ko SG, Choi HY, Oh MS, Park W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol. 2009; 125:286–290.14. Mir-Palomo S, Nácher A, Díez-Sales O, Ofelia Vila Busó MA, Caddeo C, Manca ML, Manconi M, Fadda AM, Saurí AR. Inhibition of skin inflammation by baicalin ultradeformable vesicles. Int J Pharm. 2016; 511:23–29.15. Jung HS, Kim MH, Gwak NG, Im YS, Lee KY, Sohn Y, Choi H, Yang WM. Antiallergic effects of Scutellaria baicalensis on inflammation in vivo and in vitro. J Ethnopharmacol. 2012; 141:345–349.16. Kim OS, Seo CS, Kim Y, Shin HK, Ha H. Extracts of Scutellariae Radix inhibit low-density lipoprotein oxidation and the lipopolysaccharide-induced macrophage inflammatory response. Mol Med Rep. 2015; 12:1335–1341.17. Choi YH, Han EH, Chai OH, Kim YK, Kim HT, Song CH. Scutellaria baicalensis inhibits mast cell-mediated anaphylactic reactions. Korean J Phys Anthropol. 2010; 23:217–227.18. Liu T, Dai W, Li C, Liu F, Chen Y, Weng D, Chen J. Baicalin alleviates silica-induced lung inflammation and fibrosis by inhibiting the Th17 response in C57BL/6 mice. J Nat Prod. 2015; 78:3049–3057.19. He X, Wei Z, Zhou E, Chen L, Kou J, Wang J, Yang Z. Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Int Immunopharmacol. 2015; 28:470–476.20. Nakajima T, Imanishi M, Yamamoto K, Cyong JC, Hirai K. Inhibitory effect of baicalein, a flavonoid in Scutellaria root, on eotaxin production by human dermal fibroblasts. Planta Med. 2001; 67:132–135.21. Mabalirajan U, Ahmad T, Rehman R, Leishangthem GD, Dinda AK, Agrawal A, Ghosh B, Sharma SK. Baicalein reduces airway injury in allergen and IL-13 induced airway inflammation. PLoS One. 2013; 8:e62916.22. Ryu EK, Kim TH, Jang EJ, Choi YS, Kim ST, Hahm KB, Lee HJ. Wogonin, a plant flavone from Scutellariae radix, attenuated ovalbumin-induced airway inflammation in mouse model of asthma via the suppression of IL-4/STAT6 signaling. J Clin Biochem Nutr. 2015; 57:105–112.23. Shin HS, Bae MJ, Choi DW, Shon DH. Skullcap (Scutellaria baicalensis) extract and its active compound, wogonin, inhibit ovalbumin-induced Th2-mediated response. Molecules. 2014; 19:2536–2545.24. Chai OH, Han EH, Lee HK, Song CH. Mast cells play a key role in Th2 cytokine-dependent asthma model through production of adhesion molecules by liberation of TNF-α. Exp Mol Med. 2011; 43:35–43.25. Chai OH, Shon DH, Han EH, Kim HT, Song CH. Effects of Anemarrhena asphodeloides on IgE-mediated passive cutaneous anaphylaxis, compound 48/80-induced systemic anaphylaxis and mast cell activation. Exp Toxicol Pathol. 2013; 65:419–426.26. Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016; 28:163–171.27. Cosmi L, Maggi L, Santarlasci V, Liotta F, Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014; 85:36–42.28. Winbery SL, Lieberman PL. Histamine and antihistamines in anaphylaxis. Clin Allergy Immunol. 2002; 17:287–317.29. Busse WW, Rosenwasser LJ. Mechanisms of asthma. J Allergy Clin Immunol. 2003; 111:3 Suppl. S799–S804.30. Li HB, Jiang Y, Chen F. Separation methods used for Scutellaria baicalensis active components. J Chromatogr B Analyt Technol Biomed Life Sci. 2004; 812:277–290.31. Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009; 35:57–68.32. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454.33. Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness: a murine model. Allergy. 1999; 54:297–305.34. Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005; 115:459–465.35. Guo HW, Yun CX, Hou GH, Du J, Huang X, Lu Y, Keller ET, Zhang J, Deng JG. Mangiferin attenuates TH1/TH2 cytokine imbalance in an ovalbumin-induced asthmatic mouse model. PLoS One. 2014; 9:e100394.36. Moiseeva EP, Bradding P. Mast cells in lung inflammation. Adv Exp Med Biol. 2011; 716:235–269.37. White MV. The role of histamine in allergic diseases. J Allergy Clin Immunol. 1990; 86(4 Pt 2):599–605.38. Rafferty P, Beasley R, Holgate ST. The contribution of histamine to immediate bronchoconstriction provoked by inhaled allergen and adenosine 5' monophosphate in atopic asthma. Am Rev Respir Dis. 1987; 136:369–373.39. Taylor-Clark TE. Insights into the mechanisms of histamine-induced inflammation in the nasal mucosa. Pulm Pharmacol Ther. 2008; 21:455–460.40. Nelson HS. Prospects for antihistamines in the treatment of asthma. J Allergy Clin Immunol. 2003; 112:4 Suppl. S96–S100.41. Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J Immunol. 1993; 151:3460–3466.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Scutellaria baicalensis Inhibits Mast Cell-Mediated Anaphylactic Reactions
- Effects of cortex mori on the compound 48/80-induced anaphylactic shock and histamine release from mast cells
- Anti-Allergic Effect of Oroxylin A from Oroxylum indicum Using in vivo and in vitro Experiments
- Olopatadine ophthalmic solution suppresses substance P release in the conjunctivitis models
- Stem cell therapy in animal models of allergic airway diseases