Asia Pac Allergy.
2012 Apr;2(2):115-121. 10.5415/apallergy.2012.2.2.115.
Olopatadine ophthalmic solution suppresses substance P release in the conjunctivitis models
- Affiliations
-
- 1Marketing Department Sales & Marketing Division and Pharmacological Research Laboratories, Research Division, Kyowa Hakko Kirin Co., Ltd., Tokyo 100-8185, Japan. tadafumi.tamura@kyowa-kirin.co.jp
- KMID: 2397364
- DOI: http://doi.org/10.5415/apallergy.2012.2.2.115
Abstract
- BACKGROUND
Olopatadine hydrochloride ophthalmic solutions are treated for allergic conjunctival diseases that are a selective histamine H1 receptor antagonist and an inhibitor of the release of mediators including histamine from the human mast cells. Substance P (SP) levels are increased in tears of patients with allergic conjunctivitis. However, little is known about the regulation of SP release by anti-allergic ophthalmic solutions.
OBJECTIVE
We investigated that the effect of olopatadine hydrochloride ophthalmic solutions (olopatadine 0.1% and olopatadine 0.2%) on rat conjunctivitis models compared with other anti-allergic ophthalmic solutions.
METHODS
Conjunctivitis was induced by subconjunctival injection of histamine or intravenous injection of ovalbumin in rats passively sensitized with anti-ovalbumin anti-serum. The releases of SP were determined in the conjunctiva and tears using rat antigen-induced conjunctivitis models.
RESULTS
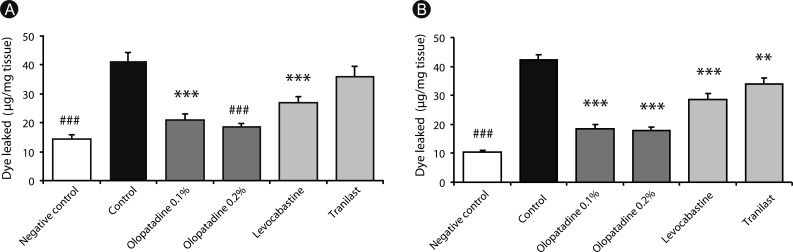
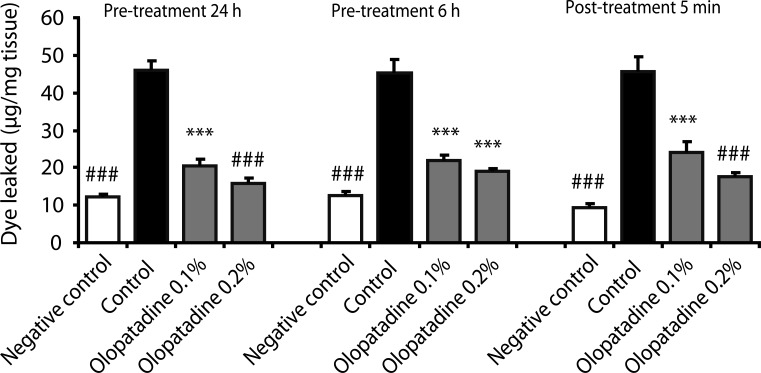
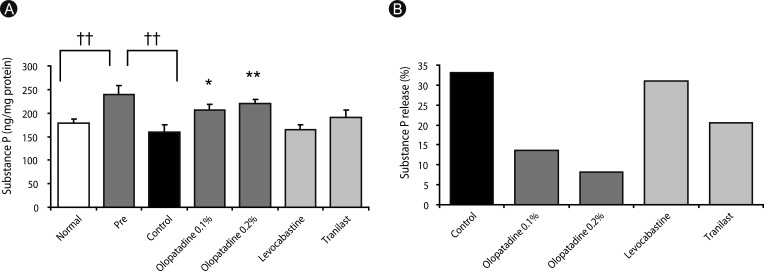
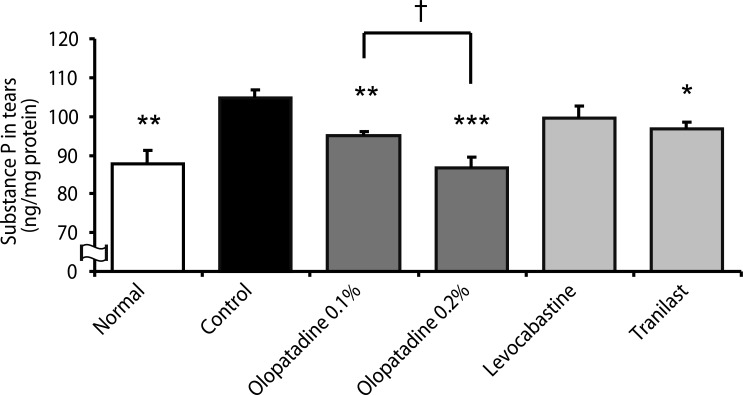
Olopatadine 0.1% and 0.2% significantly inhibited the increased conjunctival dye leaked in the histamine- or antigen-induced hyperpermeability. The inhibitory effects by olopatadine were more potent than by other tested anti-allergic ophthalmic solutions. Moreover, olopatadine significantly inhibited the release of SP from the conjunctiva.
CONCLUSION
These results indicate that olopatadine ophthalmic solutions appear to exert additional SP release inhibition besides dual-action such as selective histamine H1 receptor antagonistic action and mast cell stabilization action.
MeSH Terms
-
Animals
Conjunctiva
Conjunctival Diseases
Conjunctivitis*
Conjunctivitis, Allergic
Histamine
Humans
Injections, Intravenous
Mast Cells
Olopatadine Hydrochloride*
Ophthalmic Solutions
Ovalbumin
Rats
Receptors, Histamine H1
Substance P*
Tears
Histamine
Olopatadine Hydrochloride
Ophthalmic Solutions
Ovalbumin
Receptors, Histamine H1
Substance P
Figure
Cited by 1 articles
-
Asia Pacific Allergy: a successful first year and the future
Yoon-Seok Chang
Asia Pac Allergy. 2012;2(2):91-92. doi: 10.5415/apallergy.2012.2.2.91.
Reference
-
1. Sharif NA, Xu SX, Miller ST, Gamache DA, Yanni JM. Characterization of the ocular antiallergic and antihistaminic effects of olopatadine (AL-4943A), a novel drug for treating ocular allergic diseases. J Pharmacol Exp Ther. 1996; 278:1252–1261. PMID: 8819509.2. Yanni JM, Stephens DJ, Miller ST, Weimer LK, Graff G, Parnell D, Lang LS, Spellman JM, Brady MT, Gamache DA. The in vitro and in vivo ocular pharmacology of olopatadine (AL-4943A), an effective anti-allergic/antihistaminic agent. J Ocul Pharmacol Ther. 1996; 12:389–400. PMID: 8951675.3. Yanni JM, Miller ST, Gamache DA, Spellman JM, Xu S, Sharif NA. Comparative effects of topical ocular anti-allergy drugs on human conjunctival mast cells. Ann Allergy Asthma Immunol. 1997; 79:541–545. PMID: 9433371.
Article4. Yanni JM, Weimer LK, Sharif NA, Xu SX, Gamache DA, Spellman JM. Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch Ophthalmol. 1999; 117:643–647. PMID: 10326962.
Article5. Fujishima H, Takeyama M, Takeuchi T, Saito I, Tsubota K. Elevated levels of substance P in tears of patients with allergic conjunctivitis and vernal keratoconjunctivitis. Clin Exp Allergy. 1997; 27:372–378. PMID: 9146929.
Article6. Micera A, Lambiase A, Bonini S. The role of neuromediators in ocular allergy. Curr Opin Allergy Clin Immunol. 2008; 8:466–471. PMID: 18769203.
Article7. Mantelli F, Micera A, Sacchetti M, Bonini S. Neurogenic inflammation of the ocular surface. Curr Opin Allergy Clin Immunol. 2010; 10:498–504. PMID: 20706114.
Article8. Rossi R, Johansson O. Cutaneous innervation and the role of neuronal peptides in cutaneous inflammation: a minireview. Eur J Dermatol. 1998; 8:299–306. PMID: 9683879.9. Raap U, Ständer S, Metz M. Pathophysiology of itch and new treatments. Curr Opin Allergy Clin Immunol. 2011; 11:420–427. PMID: 21772138.
Article10. Tamura T, Amano T, Ohmori K, Manabe H. The effects of olopatadine hydrochloride on the number of scratching induced by repeated application of oxazolone in mice. Eur J Pharmacol. 2005; 524:149–154. PMID: 16259975.
Article11. Murota H, El-latif MA, Tamura T, Amano T, Katayama I. Olopatadine hydrochloride improves dermatitis score and inhibits scratch behavior in NC/Nga mice. Int Arch Allergy Immunol. 2010; 153:121–132. PMID: 20407268.
Article12. Stotland LM, Share NN. Active bronchial anaphylaxis in the rat. Can J Physiol Pharmacol. 1974; 52:1114–1118. PMID: 4375539.
Article13. Minami K, Hossen MA, Kamei C. Increasing effect by simultaneous use of levocabastine and pemirolast on experimental allergic conjunctivitis in rats. Biol Pharm Bull. 2005; 28:473–476. PMID: 15744071.
Article14. Shimura M, Yasuda K, Miyazawa A, Otani T, Nakazawa T. Pre-seasonal treatment with topical olopatadine suppresses the clinical symptoms of seasonal allergic conjunctivitis. Am J Ophthalmol. 2011; 151:697–702.e2. PMID: 21257151.
Article15. Sharif NA, Xu SX, Yanni JM. Olopatadine (AL-4943A): ligand binding and functional studies on a novel, long acting H1-selective histamine antagonist and anti-allergic agent for use in allergic conjunctivitis. J Ocul Pharmacol Ther. 1996; 12:401–407. PMID: 8951676.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Stabilization of Human Umbilical Cord Blood-Derived Mast Cells in Accordance with Ketotifen and Olopatadine Concentration
- Comparison of the Toxicity of Olopatadine Anti-allergic Ophthalmic Agents on Rabbit Conjunctival Cells
- Effects of Mitomycin C on Trabeculectomy Outcomes in Patients Who Preoperatively Used Prostaglandin Ophthalmic Solution
- Clinical Effect of Levocabastine Eye Drops on Allergic Conjunctivitis
- The Diagnosis of Conjunctivitis