Nutr Res Pract.
2017 Oct;11(5):365-372. 10.4162/nrp.2017.11.5.365.
Effects of ingredients of Korean brown rice cookies on attenuation of cholesterol level and oxidative stress in high-fat diet-fed mice
- Affiliations
-
- 1Department of Food Science and Nutrition and Kimchi Research Institute, Pusan National University, 2 Busandaehak-ro 63 beon-gil, Geumjeong-gu, Busan 46241, Korea. yosong@pusan.ac.kr
- KMID: 2390128
- DOI: http://doi.org/10.4162/nrp.2017.11.5.365
Abstract
- BACKGROUND/OBJECTIVES
Owing to health concerns related to the consumption of traditional snacks high in sugars and fats, much effort has been made to develop functional snacks with low calorie content. In this study, a new recipe for Korean rice cookie, dasik, was developed and its antioxidative, lipid-lowering, and anti-inflammatory effects and related mechanisms were elucidated. The effects were compared with those of traditional rice cake dasik (RCD), the lipid-lowering effect of which is greater than that of traditional western-style cookies.
MATERIALS/METHODS
Ginseng-added brown rice dasik (GBRD) was prepared with brown rice flour, fructooligosaccharide, red ginseng extract, and propolis. Mice were grouped (n = 7 per group) into those fed a normal AIN-76 diet, a high-fat diet (HFD), and HFD supplemented with RCD or GBRD. Dasik in the HFD accounted for 7% of the total calories. The lipid, reactive oxygen species, and peroxynitrite levels, and degree of lipid peroxidation in the plasma or liver were determined. The expression levels of proteins involved in lipid metabolism and inflammation, and those of antioxidant enzymes were determined by western blot analysis.
RESULTS
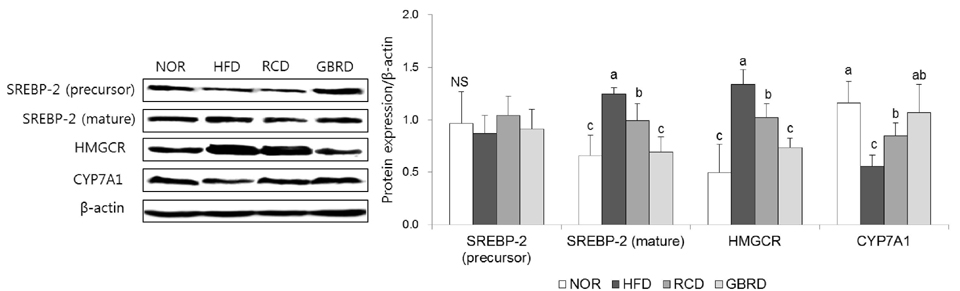
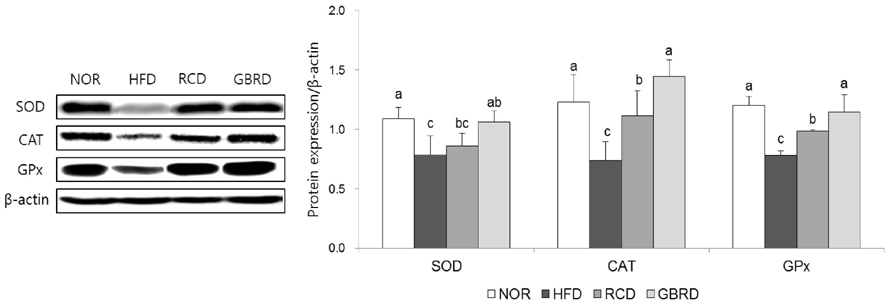
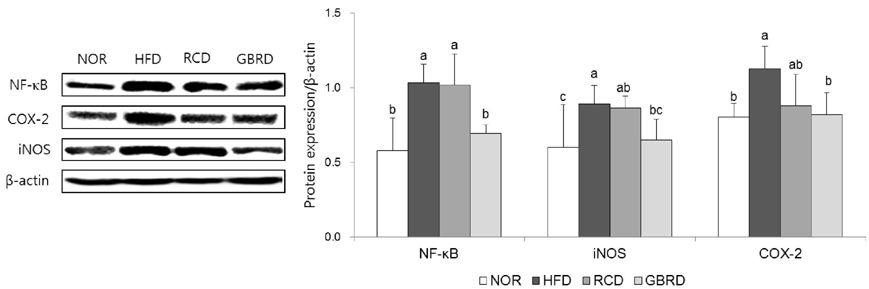
The plasma and hepatic total cholesterol concentrations in the GBRD group were significantly decreased via downregulation of sterol regulatory element-binding protein-2 and 3-hydroxy-3-methylglutaryl-CoA reductase (P < 0.05). The hepatic peroxynitrite level was significantly lower, whereas glutathione was higher, in the GBRD group than in the RCD group. Among the antioxidant enzymes, catalase (CAT) and glutathione peroxidase (GPx) were significantly upregulated in the GBRD group (P < 0.05). In addition, nuclear factor-kappaB (NF-κB) expression in the GBRD group was significantly lower than that in the RCD group.
CONCLUSIONS
GBRD decreases the plasma and hepatic cholesterol levels by downregulating cholesterol synthesis. This new dasik recipe also improves the antioxidative and anti-inflammatory status in HFD-fed mice via CAT and GPx upregulation and NF-κB downregulation. These effects were significantly higher than those of RCD.
Keyword
MeSH Terms
-
Animals
Antioxidants
Blotting, Western
Carbohydrates
Catalase
Cats
Cholesterol*
Diet
Diet, High-Fat
Down-Regulation
Fats
Flour
Glutathione
Glutathione Peroxidase
Inflammation
Lipid Metabolism
Lipid Peroxidation
Liver
Mice*
Oxidative Stress*
Oxidoreductases
Panax
Peroxynitrous Acid
Plasma
Propolis
Reactive Oxygen Species
Snacks
Sterol Regulatory Element Binding Protein 2
Up-Regulation
Antioxidants
Carbohydrates
Catalase
Cholesterol
Fats
Glutathione
Glutathione Peroxidase
Oxidoreductases
Peroxynitrous Acid
Propolis
Reactive Oxygen Species
Sterol Regulatory Element Binding Protein 2
Figure
Reference
-
1. Hong SH, Kim M, Woo M, Song YO. Rice cookie decreases plasma and hepatic lipid levels in high-fat diet-fed mice: a comparison study with traditional western style cookies. J Food Nutr Res. 2017; 5:451–457.
Article2. Hong SH, Kim M, Woo M, Noh JS, Lee J, Chung L, Song YO. The amelioration of plasma lipids by Korean traditional confectionery in middle-aged women: a cross-over study with western cookie. Nutr Res Pract. 2016; 10:590–596.
Article3. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010; 23:65–134.
Article4. Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sánchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007; 86:899–906.5. Dominguez AL, Rodrigues LR, Lima NM, Teixeira JA. An overview of the recent developments on fructooligosaccharide production and applications. Food Bioproc Tech. 2014; 7:324–337.
Article6. Delgado GT, Tamashiro WM, Junior MR, Moreno YM, Pastore GM. The putative effects of prebiotics as immunomodulatory agents. Food Res Int. 2011; 44:3167–3173.
Article7. Merino-Aguilar H, Arrieta-Baez D, Jiménez-Estrada M, Magos-Guerrero G, Hernández-Bautista RJ, Susunaga-Notario Adel C, Almanza-Pérez JC, Blancas-Flores G, Román-Ramos R, Alarcón-Aguilar FJ. Effect of fructooligosaccharides fraction from Psacalium decompositum on inflammation and dyslipidemia in rats with fructose-induced obesity. Nutrients. 2014; 6:591–604.
Article8. Nakamura Y, Natsume M, Yasuda A, Ishizaka M, Kawahata K, Koga J. Fructooligosaccharides suppress high-fat diet-induced fat accumulation in C57BL/6J mice. Biofactors. 2017; 43:145–151.
Article9. Han JY, Ahn SY, Oh EH, Nam SY, Hong JT, Oh KW, Lee MK. Red ginseng extract attenuates kainate-induced excitotoxicity by antioxidative effects. Evid Based Complement Alternat Med. 2012; 2012:479016.
Article10. Yu T, Rhee MH, Lee J, Kim SH, Yang Y, Kim HG, Kim Y, Kim C, Kwak YS, Kim JH, Cho JY. Ginsenoside Rc from Korean red ginseng (Panax ginseng C.A. Meyer) attenuates inflammatory symptoms of gastritis, hepatitis and arthritis. Am J Chin Med. 2016; 44:595–615.
Article11. Nagar H, Choi S, Jung SB, Jeon BH, Kim CS. Rg3-enriched Korean red ginseng enhances blood pressure stability in spontaneously hypertensive rats. Integr Med Res. 2016; 5:223–229.
Article12. Song YB, An YR, Kim SJ, Park HW, Jung JW, Kyung JS, Hwang SY, Kim YS. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J Sci Food Agric. 2012; 92:388–396.
Article13. Bang H, Kwak JH, Ahn HY, Shin DY, Lee JH. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food. 2014; 17:128–134.
Article14. Kim EJ, Kwon KA, Lee YE, Kim JH, Kim SH, Kim JH. Korean red ginseng extract reduces hypoxia-induced epithelial-mesenchymal transition by repressing NF-κB and ERK1/2 pathways in colon cancer. J Ginseng Res. Forthcoming 2017.
Article15. Watts GF, Burke V. Lipid-lowering trials in the primary and secondary prevention of coronary heart disease: new evidence, implications and outstanding issues. Curr Opin Lipidol. 1996; 7:341–355.
Article16. Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, Garelli P, Casini A, Manco M, Mingrone G, Risaliti A, Frega GN, Benedetti A, Gastaldelli A. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011; 31:1285–1297.
Article17. Peairs AT, Rankin JW. Inflammatory response to a high-fat, low-carbohydrate weight loss diet: effect of antioxidants. Obesity (Silver Spring). 2008; 16:1573–1578.
Article18. Sinha-Hikim I, Sinha-Hikim AP, Shen R, Kim HJ, French SW, Vaziri ND, Crum AC, Rajavashisth TB, Norris KC. A novel cystine based antioxidant attenuates oxidative stress and hepatic steatosis in diet-induced obese mice. Exp Mol Pathol. 2011; 91:419–428.
Article19. Yogalakshmi B, Bhuvaneswari S, Sreeja S, Anuradha CV. Grape seed proanthocyanidins and metformin act by different mechanisms to promote insulin signaling in rats fed high calorie diet. J Cell Commun Signal. 2014; 8:13–22.
Article20. Raval J, Lyman S, Nitta T, Mohuczy D, Lemasters JJ, Kim JS, Behrns KE. Basal reactive oxygen species determine the susceptibility to apoptosis in cirrhotic hepatocytes. Free Radic Biol Med. 2006; 41:1645–1654.
Article21. Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002; 30:620–650.
Article22. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article23. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
Article24. Teare JP, Punchard NA, Powell JJ, Lumb PJ, Mitchell WD, Thompson RP. Automated spectrophotometric method for determining oxidized and reduced glutathione in liver. Clin Chem. 1993; 39:686–689.
Article25. Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med. 1994; 16:149–156.
Article26. Jung K, Hong SH, Kim M, Han JS, Jang MS, Song YO. Antiatherogenic effects of Korean cabbage kimchi with added short arm octopus. Food Sci Biotechnol. 2015; 24:249–255.
Article27. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002; 109:1125–1131.
Article28. Cao K, Xu J, Zou X, Li Y, Chen C, Zheng A, Li H, Li H, Szeto IM, Shi Y, Long J, Liu J, Feng Z. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic Biol Med. 2014; 67:396–407.
Article29. Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005; 54:1314–1323.
Article30. Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997; 89:331–340.
Article31. Crabb DW, Liangpunsakul S. Alcohol and lipid metabolism. J Gastroenterol Hepatol. 2006; 21:Suppl 3. S56–S60.
Article32. Wong J, Quinn CM, Brown AJ. SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligands for LXR. Biochem J. 2006; 400:485–491.
Article33. Yang JL, Kim YH, Lee HS, Lee MS, Moon YK. Barley β-glucan lowers serum cholesterol based on the up-regulation of cholesterol 7α-hydroxylase activity and mRNA abundance in cholesterol-fed rats. J Nutr Sci Vitaminol (Tokyo). 2003; 49:381–387.
Article34. Rong N, Ausman LM, Nicolosi RJ. Oryzanol decreases cholesterol absorption and aortic fatty streaks in hamsters. Lipids. 1997; 32:303–309.
Article35. Miura D, Ito Y, Mizukuchi A, Kise M, Aoto H, Yagasaki K. Hypocholesterolemic action of pre-germinated brown rice in hepatoma-bearing rats. Life Sci. 2006; 79:259–264.
Article36. Shen L, Xiong Y, Wang DQ, Howles P, Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC, Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013; 54:1430–1438.
Article37. Quan HY, Yuan HD, Jung MS, Ko SK, Park YG, Chung SH. Ginsenoside Re lowers blood glucose and lipid levels via activation of AMP-activated protein kinase in HepG2 cells and high-fat diet fed mice. Int J Mol Med. 2012; 29:73–80.
Article38. Park JH, Lee JY, Yeo JY, Nam JS, Jung MH. Antihyperlipidemic effect of ginsenoside Rg1 in type 2 diabetic mice. J Life Sci. 2011; 21:932–938.
Article39. Lee S, Lee MS, Kim CT, Kim IH, Kim Y. Ginsenoside Rg3 reduces lipid accumulation with AMP-Activated Protein Kinase (AMPK) activation in HepG2 cells. Int J Mol Sci. 2012; 13:5729–5739.
Article40. Kawase A, Yamada A, Gamou Y, Tahara C, Takeshita F, Murata K, Matsuda H, Samukawa K, Iwaki M. Increased effects of ginsenosides on the expression of cholesterol 7α-hydroxylase but not the bile salt export pump are involved in cholesterol metabolism. J Nat Med. 2013; 67:545–553.
Article41. Kim CS, Park JB, Kim KJ, Chang SJ, Ryoo SW, Jeon BH. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol Sin. 2002; 23:1152–1156.42. Kim YH, Kim GH, Shin JH, Kim KS, Lim JS. Effect of Korean red ginseng on testicular tissue injury after torsion and detorsion. Korean J Urol. 2010; 51:794–799.
Article43. Kim YS, Kim YH, Noh JR, Cho ES, Park JH, Son HY. Protective effect of Korean red ginseng against aflatoxin B1-induced hepatotoxicity in rat. J Ginseng Res. 2011; 35:243–249.
Article44. Ramesh T, Kim SW, Hwang SY, Sohn SH, Yoo SK, Kim SK. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012; 32:718–726.
Article45. Sun BS, Gu LJ, Fang ZM, Wang CY, Wang Z, Lee MR, Li Z, Li JJ, Sung CK. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal. 2009; 50:15–22.
Article46. Parrado J, Miramontes E, Jover M, Márquez JC, Angeles Mejias M, Collantes de, Absi E, Bautista J. Prevention of brain protein and lipid oxidation elicited by a water-soluble oryzanol enzymatic extract derived from rice bran. Eur J Nutr. 2003; 42:307–314.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidative and antiproliferative activities of ethanol extracts from pigmented giant embryo rice (Oryza sativa L. cv. Keunnunjami) before and after germination
- Effects of caloric restriction on the expression of lipocalin-2 and its receptor in the brown adipose tissue of high-fat diet-fed mice
- Hypocholesterolemic metabolism of dietary red pericarp glutinous rice rich in phenolic compounds in mice fed a high cholesterol diet
- Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice
- Anti-hyperlipidemic activity of Rhynchosia nulubilis seeds pickled with brown rice vinegar in mice fed a high-fat diet