Korean J Hepatobiliary Pancreat Surg.
2013 Aug;17(3):89-108. 10.14701/kjhbps.2013.17.3.89.
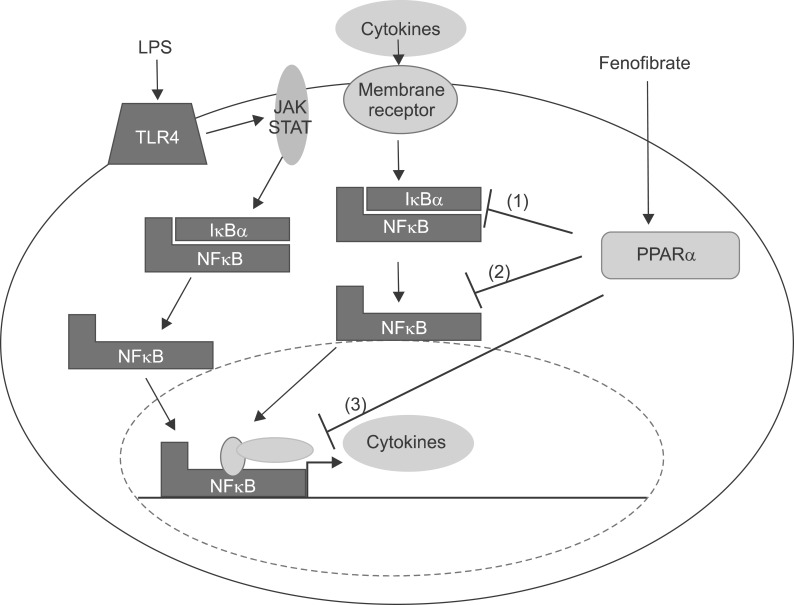
Fenofibrate, a peroxisome proliferator-activated receptor alpha-agonist, blocks lipopolysaccharide-induced inflammatory pathways in mouse liver
- Affiliations
-
- 1Department of Surgery, School of Medicine, Wonkwang University, Iksan, Korea. furufuru79@naver.com
- KMID: 1472524
- DOI: http://doi.org/10.14701/kjhbps.2013.17.3.89
Abstract
- BACKGROUNDS/AIMS
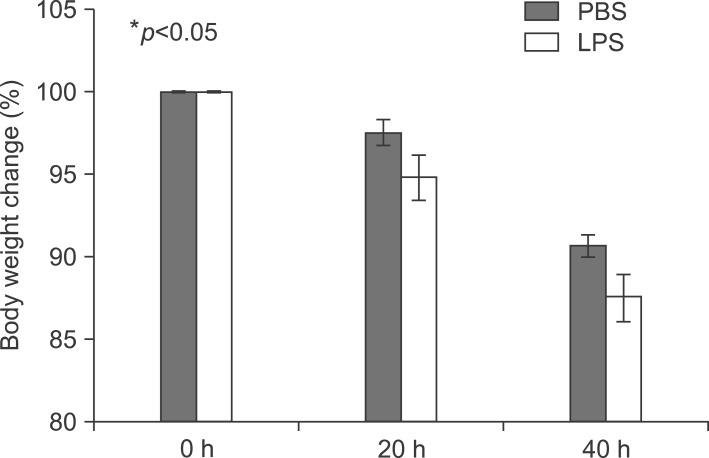
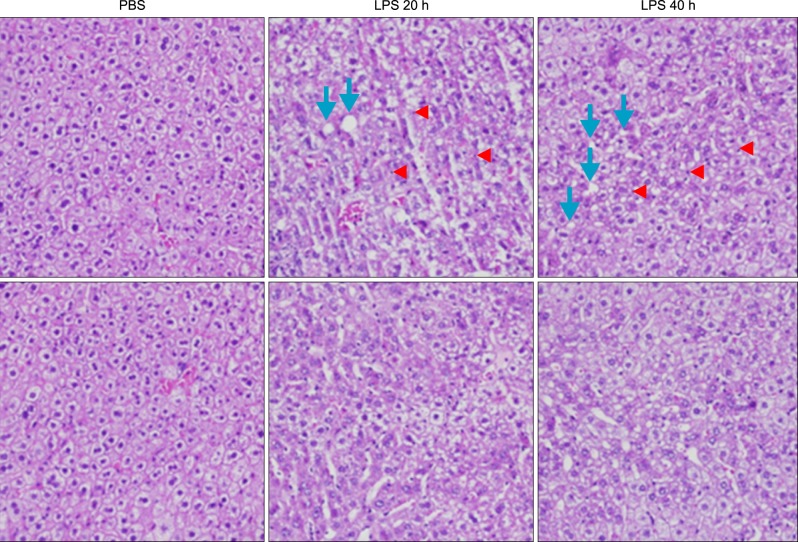
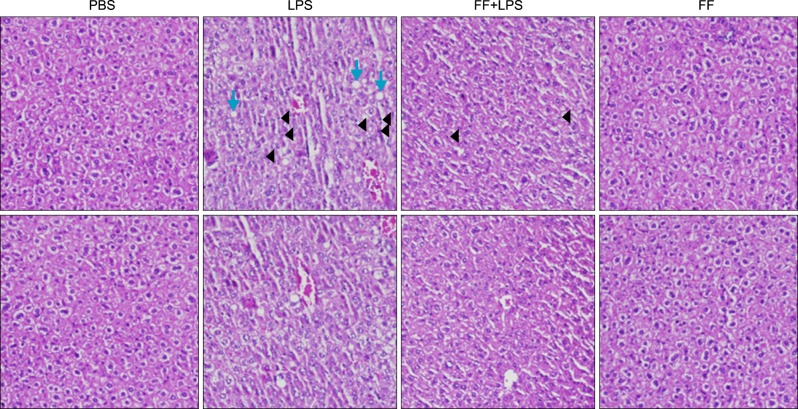
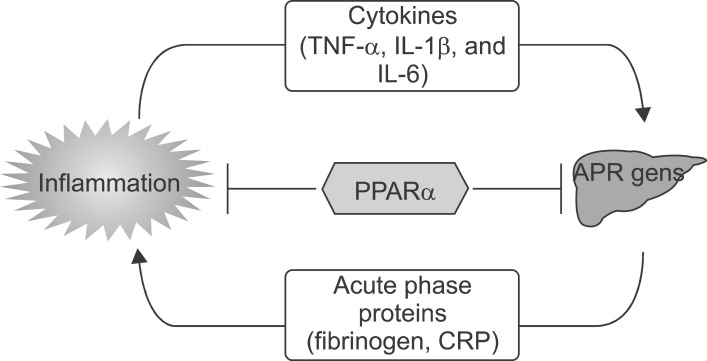
During the acute phase response, cytokines induce marked alterations in lipid metabolism including an increase in serum triglyceride levels and a decrease in hepatic fatty acid oxidation, in bile acid synthesis, and in high-density lipoprotein levels.
METHODS
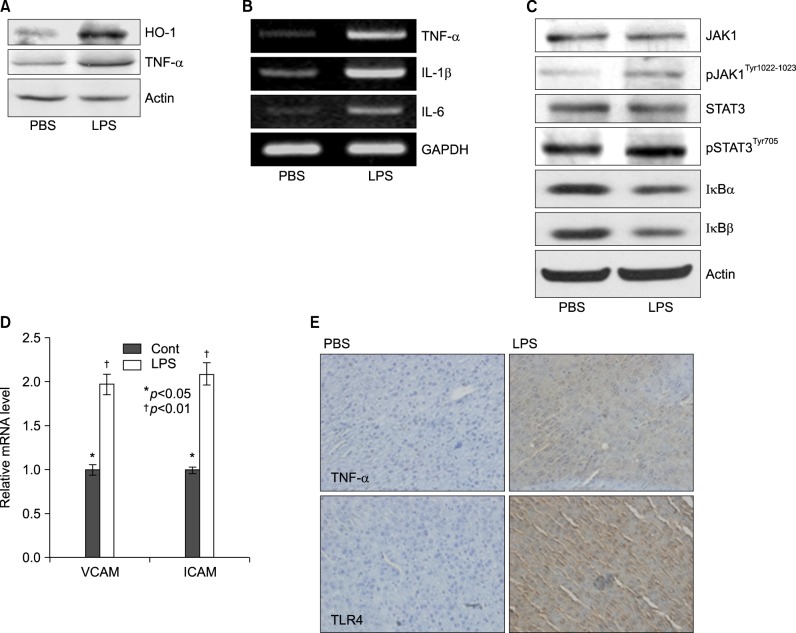
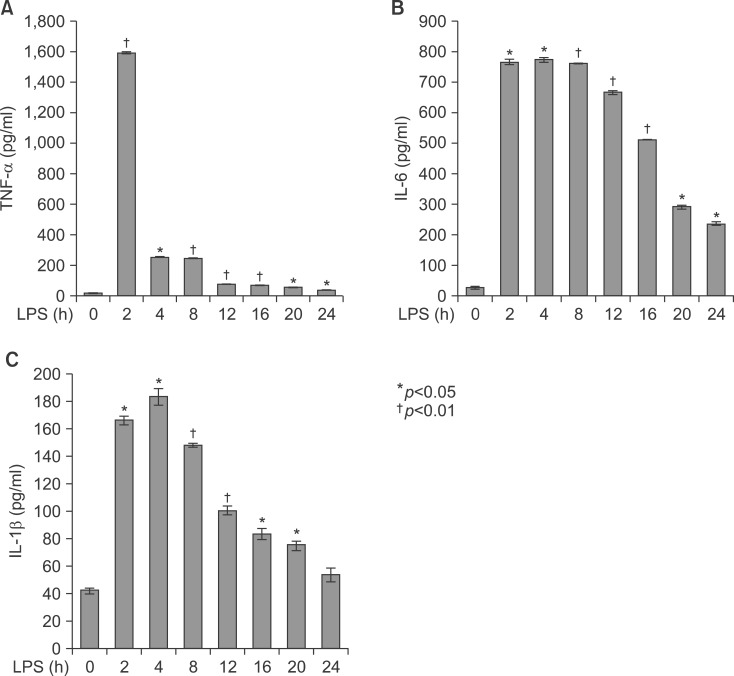
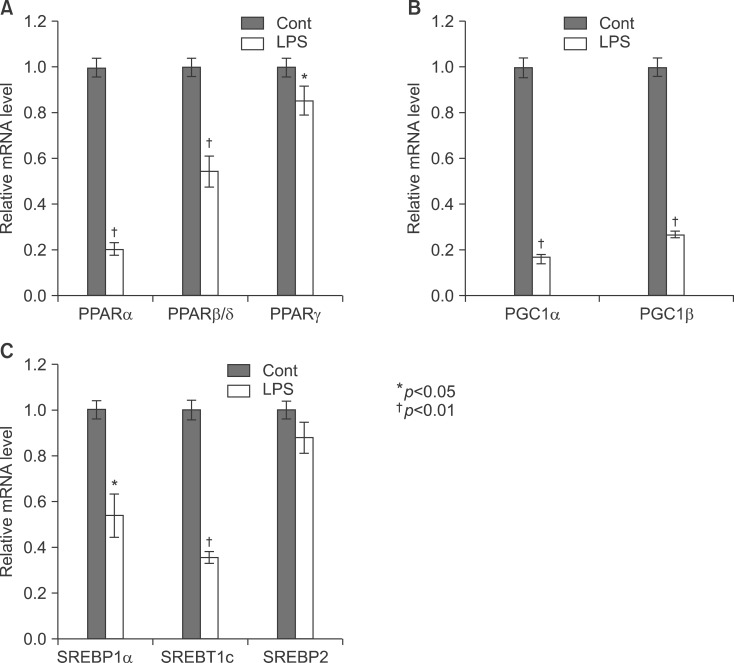
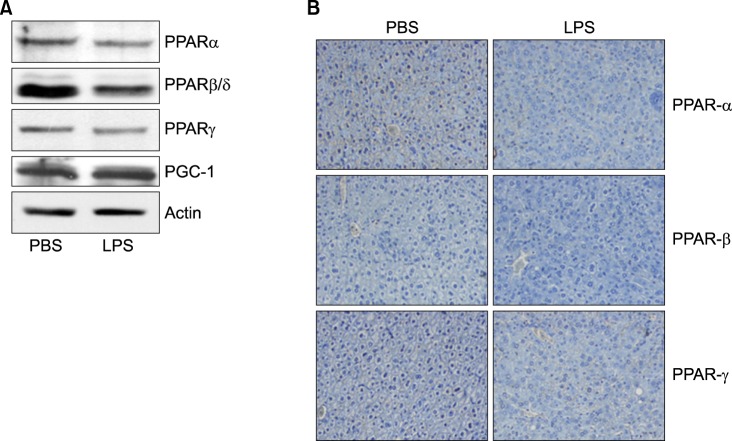
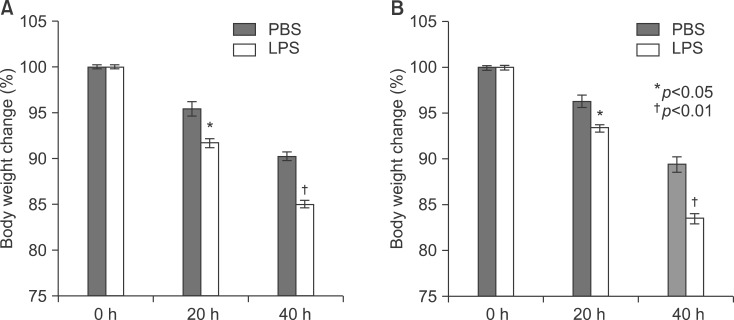
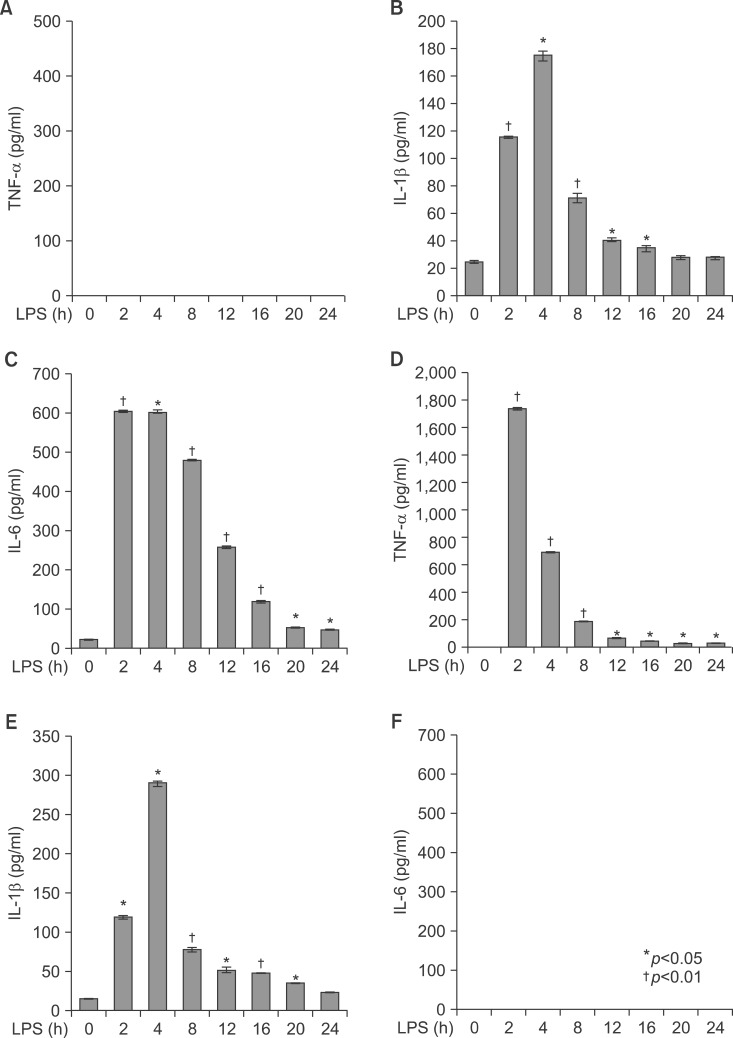
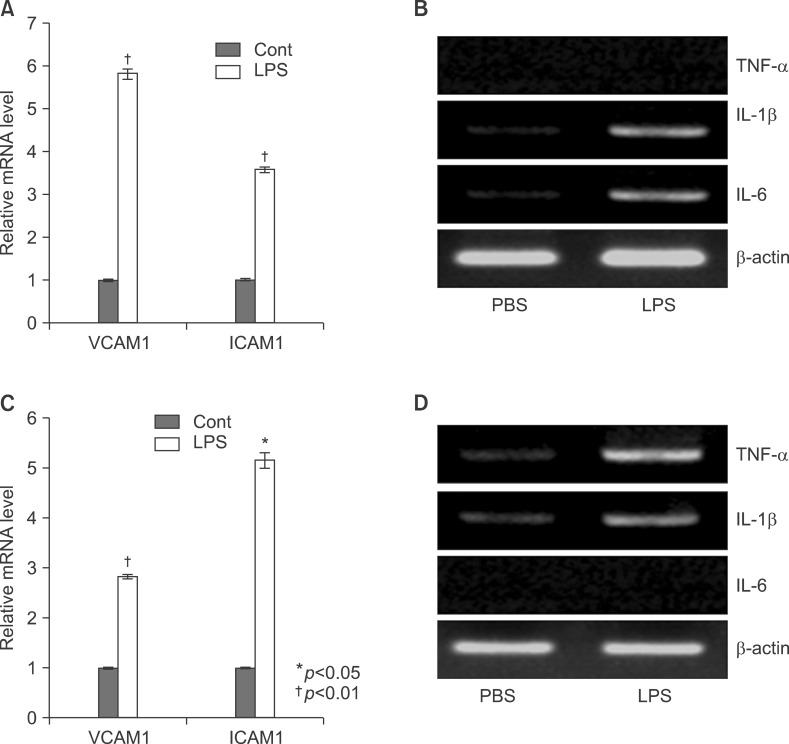
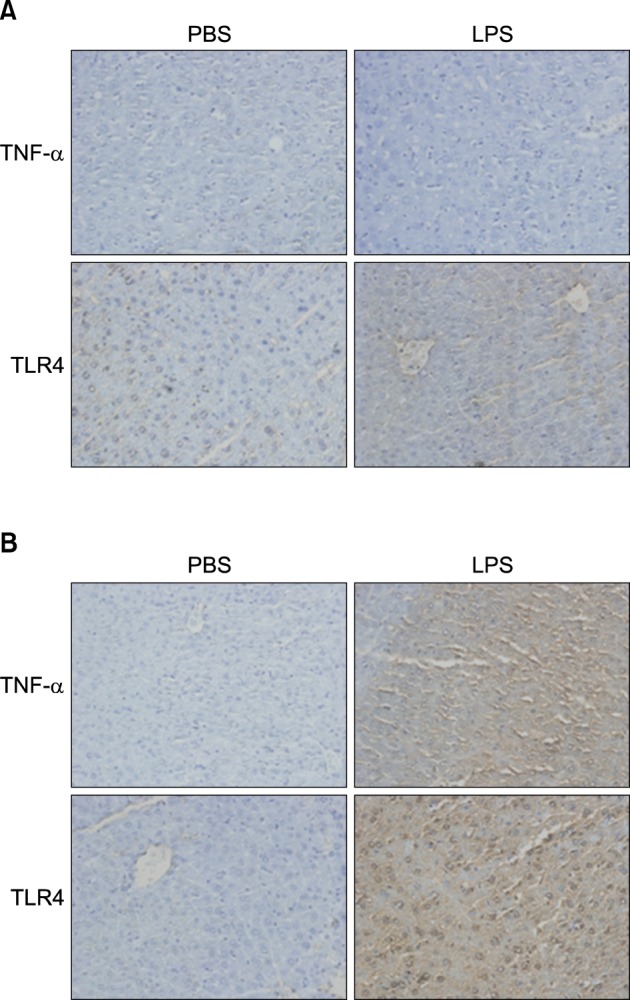
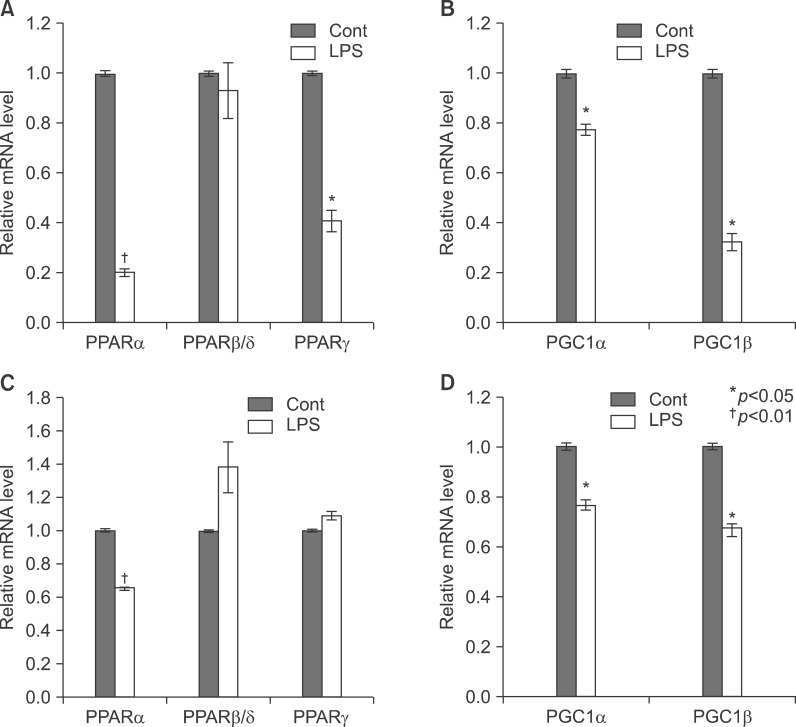
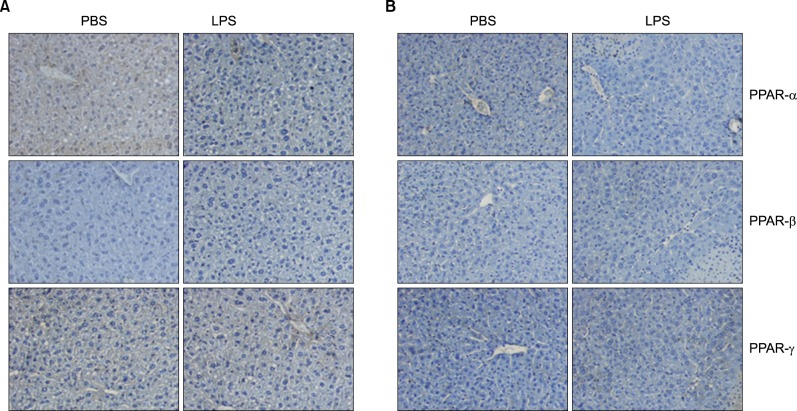
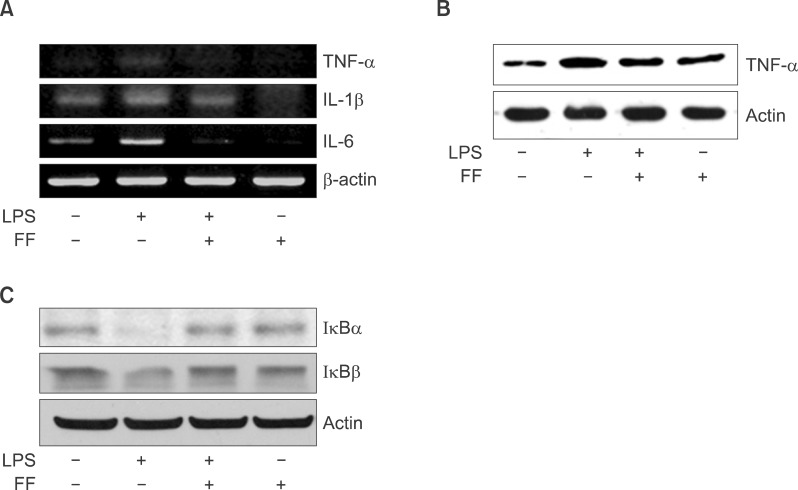
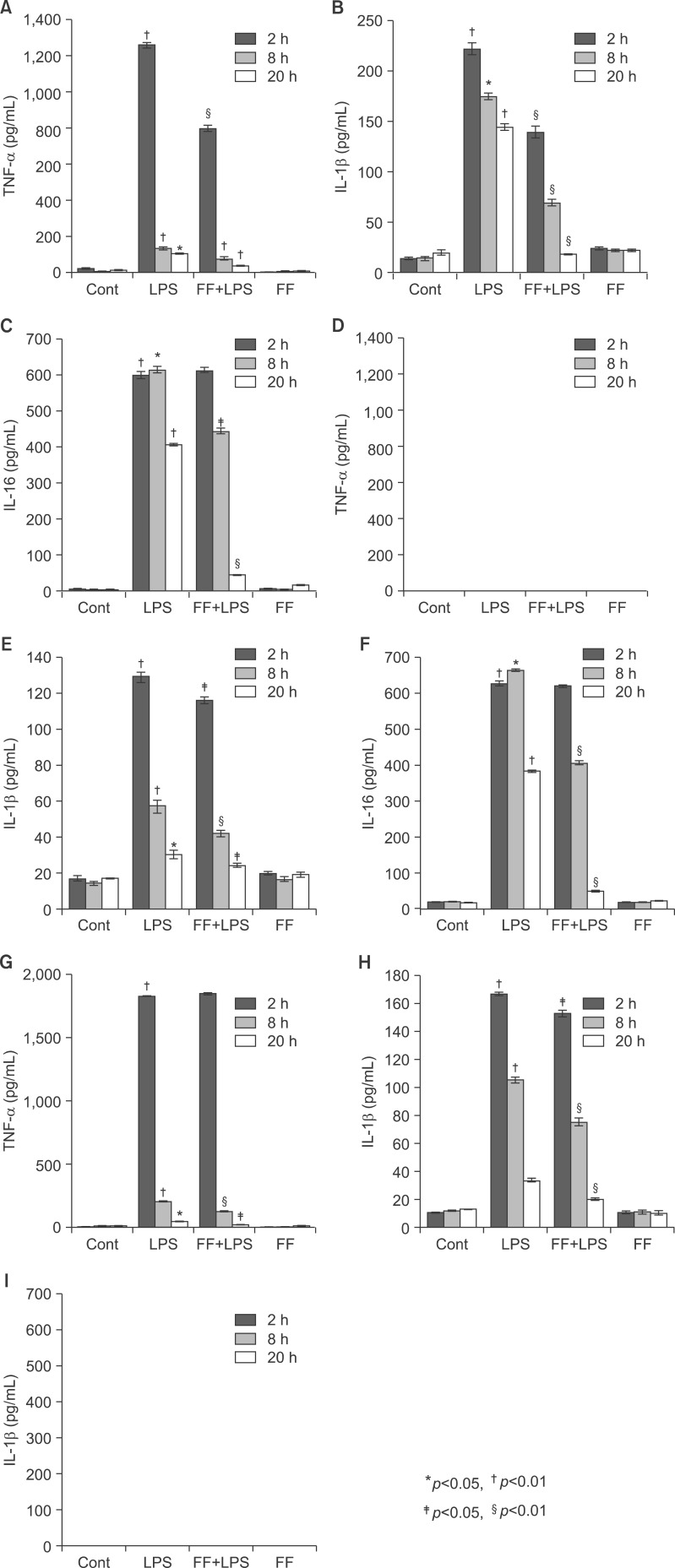
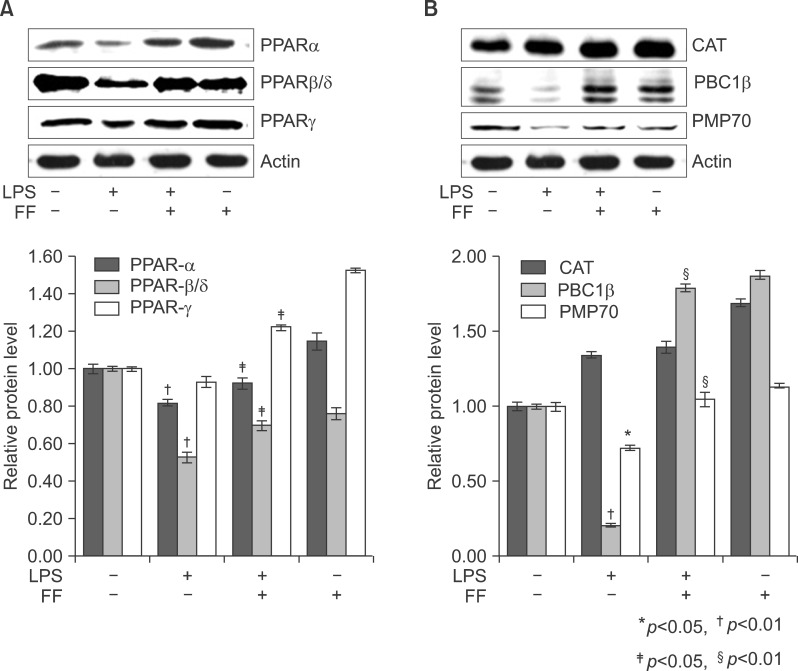
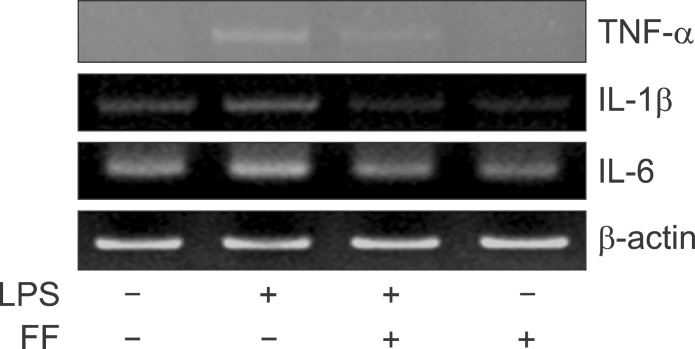
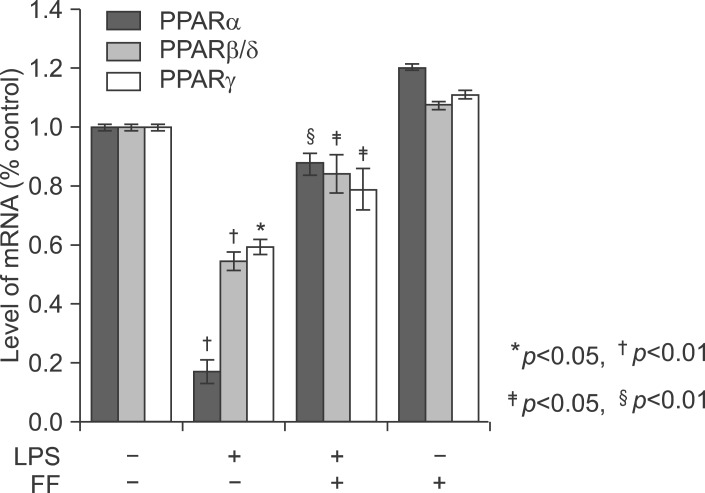
Peroxisome proliferator-activated receptors (PPARs: PPARalpha, beta/delta, and gamma) regulate fatty acid metabolism, glucose homeostasis, cell proliferation, differentiation and inflammation. Proinflammatory profiles including tumor necrosis factor alpha (TNF-alpha), interleukin-1beta (IL-1beta), and interleukin-6 (IL-6) are the important pathological factors in inflammatory responses during the pathological progression of the acute phase response. Lipopolysaccarides (LPS) induced the expression of TNF-alpha, IL-1beta, and IL-6. LPS-induced inflammation decrease the expression of peroxisome proliferator-activated receptor alpha (PPARalpha), PPARbeta/delta, PPARgamma, and coactivators PPARgamma co-activator 1 alpha (PGC-1alpha), PGC-1beta messenger RNA (mRNA) in the liver of Balb/c mouse. In addition, LPS-induced inflammation diminishes the protein level of PPARalpha, PPARbeta/delta, and PPARgamma. Proinflammatory cytokines including TNFalpha, IL-1beta, and IL-6 are the principal reducer of PPARs. However, the knockout mouse model against TNFalpha and IL-6 does not block decrease of PPARs in serum and liver. The mice were pretreated with fenofibrate at 100 mg/kg for 2 days.
RESULTS
These treatment protocols increased the amount of PPARs mRNA in the liver. Fenofibrate inhibited LPS-induced TNF-alpha, IL-1beta, and IL-6 production in the serum and liver. Similar results were obtained when human hepatoma HepG2 cells exposed to LPS were co-incubated with fenofibrate. LPS-treated HepG2 cells decreased expression of IkappaB. Moreover, activation of PPARs abrogated LPS-induced degradation of IkappaB, thus suppressing LPS-induced NF-kappaB activities.
CONCLUSIONS
Therefore, fenofibrate decreases the expression and secretion of TNF-alpha, IL-1beta, and IL-6 via the NF-kappaB signaling pathway, thus serving as therapeutic targets to attenuate inflammation that is involved in hepatic pathological progression.
MeSH Terms
-
Animals
Bile
Carcinoma, Hepatocellular
Cell Proliferation
Clinical Protocols
Cytokines
Fenofibrate
Glucose
Hep G2 Cells
Homeostasis
Humans
Inflammation
Interleukin-1beta
Interleukin-6
Lipid Metabolism
Lipoproteins
Liver
Mice
Mice, Knockout
NF-kappa B
Peroxisome Proliferator-Activated Receptors
Peroxisomes
PPAR alpha
PPAR-beta
PPAR delta
PPAR gamma
RNA, Messenger
Tumor Necrosis Factor-alpha
Cytokines
Fenofibrate
Glucose
Interleukin-1beta
Interleukin-6
Lipoproteins
NF-kappa B
PPAR alpha
PPAR-beta
PPAR delta
PPAR gamma
Peroxisome Proliferator-Activated Receptors
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Streetz KL, Wüstefeld T, Klein C, et al. Mediators of inflammation and acute phase response in the liver. Cell Mol Biol (Noisy-le-grand). 2001; 47:661–673. PMID: 11502073.2. Hoffmeister A, Rothenbacher D, Bäzner U, et al. Role of novel markers of inflammation in patients with stable coronary heart disease. Am J Cardiol. 2001; 87:262–266. PMID: 11165957.
Article3. Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001; 285:2481–2485. PMID: 11368701.4. Heinrich PC, Behrmann I, Müller-Newen G, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998; 334:297–314. PMID: 9716487.
Article5. Kim H, Baumann H. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol Cell Biol. 1999; 19:5326–5338. PMID: 10409724.
Article6. Yoo JY, Wang W, Desiderio S, et al. Synergistic activity of STAT3 and c-Jun at a specific array of DNA elements in the alpha 2-macroglobulin promoter. J Biol Chem. 2001; 276:26421–26429. PMID: 11319221.7. Zauberman A, Lapter S, Zipori D. Smad proteins suppress CCAAT/enhancer-binding protein (C/EBP) beta- and STAT3-mediated transcriptional activation of the haptoglobin promoter. J Biol Chem. 2001; 276:24719–24725. PMID: 11331273.8. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990; 347:645–650. PMID: 2129546.
Article9. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997; 94:4312–4317. PMID: 9113986.10. Gervois P, Chopin-Delannoy S, Fadel A, et al. Fibrates increase human REV-ERBalpha expression in liver via a novel peroxisome proliferator-activated receptor response element. Mol Endocrinol. 1999; 13:400–409. PMID: 10076997.11. Kersten S, Seydoux J, Peters JM, et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999; 103:1489–1498. PMID: 10359558.12. Guerre-Millo M, Gervois P, Raspé E, et al. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000; 275:16638–16642. PMID: 10828060.13. Gervois P, Torra IP, Fruchart JC, et al. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin Chem Lab Med. 2000; 38:3–11. PMID: 10774955.
Article14. Gervois P, Kleemann R, Pilon A, et al. Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-alpha activator fenofibrate. J Biol Chem. 2004; 279:16154–16160. PMID: 14764586.15. Stienstra R, Mandard S, Patsouris D, et al. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007; 148:2753–2763. PMID: 17347305.16. He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003; 100:15712–15717. PMID: 14660788.17. Marra F, Efsen E, Romanelli RG, et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000; 119:466–478. PMID: 10930382.18. Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002; 2:748–759. PMID: 12360213.
Article19. Planavila A, Rodríguez-calvo R, Jové M, et al. Peroxisome proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc Res. 2005; 65:832–841. PMID: 15721863.20. Planavila A, Laguna JC, Vázquez-carrera M. Nuclear factor-kappaB activation leads to down-regulation of fatty acid oxidation during cardiac hypertrophy. J Biol Chem. 2005; 280:17464–17471. PMID: 15728586.21. Xu J, Storer PD, Chavis JA, et al. Agonists for the peroxisome proliferator-activated receptor-alpha and the retinoid X receptor inhibit inflammatory responses of microglia. J Neurosci Res. 2005; 81:403–411. PMID: 15968640.22. Jonkers IJ, Mohrschladt MF, Westendorp RG, et al. Severe hypertriglyceridemia with insulin resistance is associated with systemic inflammation: reversal with bezafibrate therapy in a randomized controlled trial. Am J Med. 2002; 112:275–280. PMID: 11893366.
Article23. Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004; 45:1169–1196. PMID: 15102878.24. Zhong Z, Wen Z, Darnell JE Jr. Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci USA. 1994; 91:4806–4810. PMID: 7545930.
Article25. Devchand PR, Keller H, Peters JM, et al. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996; 384:39–43. PMID: 8900274.26. Jackson SM, Parhami F, Xi XP, et al. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999; 19:2094–2104. PMID: 10479650.
Article27. Marx N, Mackman N, Schönbeck U, et al. PPARalpha activators inhibit tissue factor expression and activity in human monocytes. Circulation. 2001; 103:213–219. PMID: 11208679.28. Neve BP, Corseaux D, Chinetti G, et al. PPARalpha agonists inhibit tissue factor expression in human monocytes and macrophages. Circulation. 2001; 103:207–212. PMID: 11208678.29. Sirtori CR, Colli S. Influences of lipid-modifying agents on hemostasis. Cardiovasc Drugs Ther. 1993; 7:817–823. PMID: 8110626.
Article30. Gervois P, Vu-dac N, Kleemann R, et al. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor alpha agonists via inhibition of CCAAT box/enhancer-binding protein beta. J Biol Chem. 2001; 276:33471–33477. PMID: 11418615.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulatory Effects of Fenofibrate with Inflammatory Response and Myocardiac Dysfunction in Lipopolysaccharide-stimulated Heart Tissues
- Protective Effects of Fenofibrate Against the Inflammatory Cytokines in Lipopolysaccharide-Induced Mice Brain Tissues
- Refocusing Peroxisome Proliferator Activated Receptor-alpha: A New Insight for Therapeutic Roles in Diabetes
- Peroxisomal Fitness: A Potential Protective Mechanism of Fenofibrate against High Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice
- Safety and Efficacy of Peroxisome Proliferator-Activated Receptor-alpha Agonist for Treating Cardiovascular Disease