Nutr Res Pract.
2020 Feb;14(1):3-11. 10.4162/nrp.2020.14.1.3.
Neuroprotective effects of urolithin A on Hâ‚‚Oâ‚‚-induced oxidative stress-mediated apoptosis in SK-N-MC cells
- Affiliations
-
- 1Research Institute, Seoul Medical Center, Seoul 02053, Korea. nostoi72@naver.com
- 2Department of Neurosurgery, Seoul Medical Center, 156 Shinnea-ro, Seoul 02053, Korea.
- KMID: 2468507
- DOI: http://doi.org/10.4162/nrp.2020.14.1.3
Abstract
- BACKGROUND/OBJECTIVES
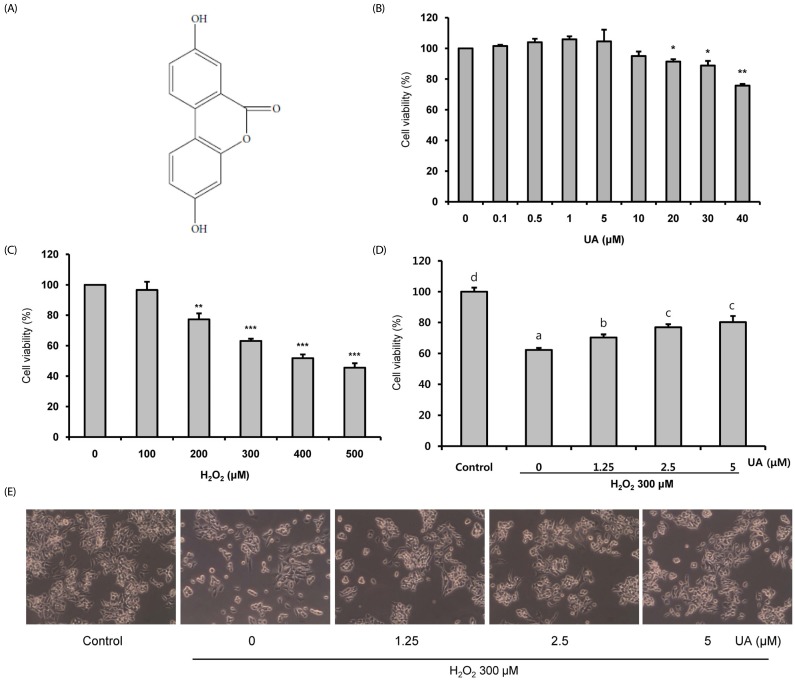
Oxidative stress causes cell damage and death, which contribute to the pathogenesis of neurodegenerative diseases. Urolithin A (UA), a gut microbial-derived metabolite of ellagitannins and ellagic acid, has high bioavailability and various health benefits such as antioxidant and anti-inflammatory effects. However, it is unknown whether it has protective effects against oxidative stress-induced cell death. We investigated whether UA ameliorates Hâ‚‚Oâ‚‚-induced neuronal cell death.
MATERIALS/METHODS
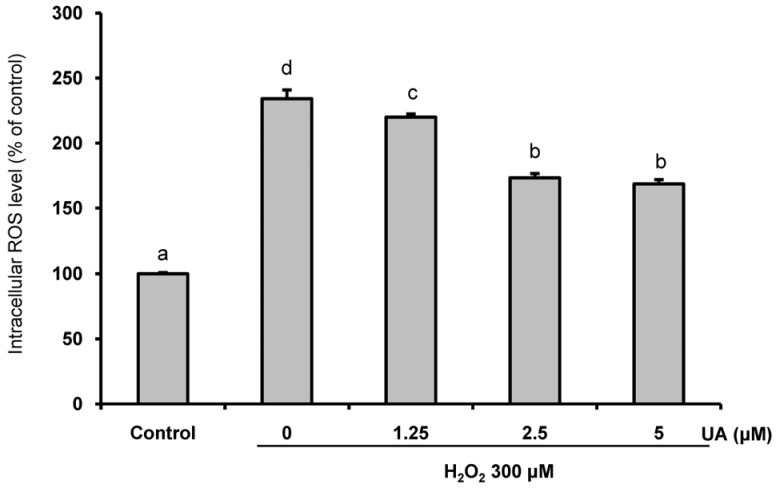
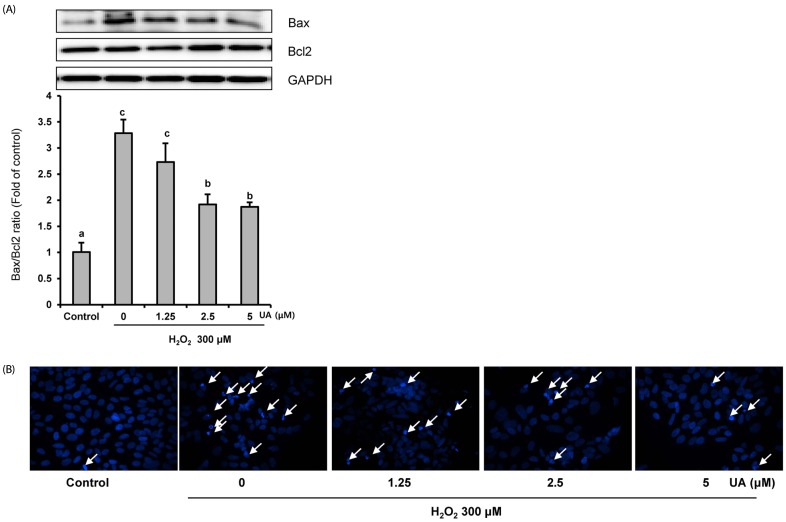
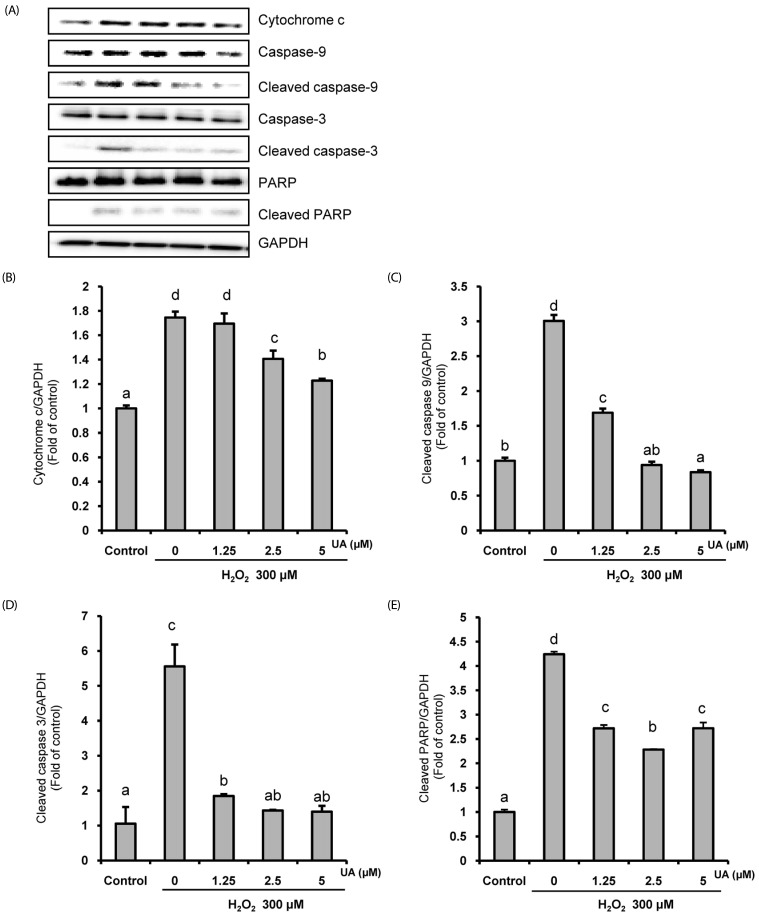
We induced oxidative damage with 300 µM Hâ‚‚Oâ‚‚ after UA pretreatment at concentrations of 1.25, 2.5, and 5 µM in SK-N-MC cells. Cytotoxicity and cell viability were determined using the CCK-8 assay. The formation of reactive oxygen species (ROS) was measured using a 2,7-dichlorofluorescein diacetate assay. Hoechst 33342 staining was used to characterize morphological changes in apoptotic cells. The expressions of apoptosis proteins were measured using Western blotting.
RESULTS
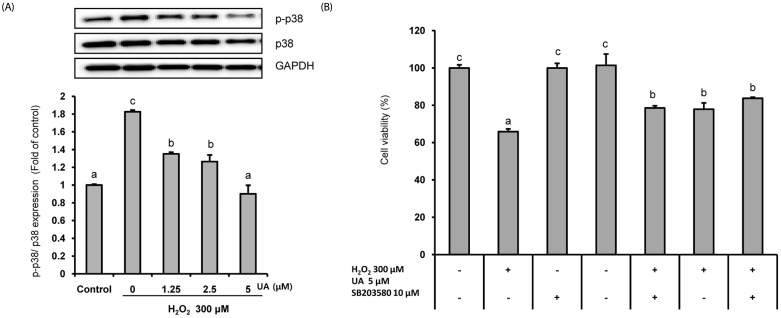
UA significantly increased cell viability and decreased intracellular ROS production in a dose-dependent manner in SK-N-MC cells. It also decreased the Bax/Bcl-2 ratio and the expressions of cytochrome c, cleaved caspase-9, cleaved caspase-3, and cleaved PARP. In addition, it suppressed the phosphorylation of the p38 mitogen-activated protein kinase (MAPK) pathway.
CONCLUSIONS
UA attenuates oxidative stress-induced apoptosis via inhibiting the mitochondrial-related apoptosis pathway and modulating the p38 MAPK pathway, suggesting that it may be an effective neuroprotective agent.
MeSH Terms
-
Apoptosis*
Biological Availability
Blotting, Western
Caspase 3
Caspase 9
Cell Death
Cell Survival
Cytochromes c
Ellagic Acid
Hydrolyzable Tannins
Insurance Benefits
Neurodegenerative Diseases
Neurons
Neuroprotective Agents*
Oxidative Stress
p38 Mitogen-Activated Protein Kinases
Phosphorylation
Protein Kinases
Reactive Oxygen Species
Sincalide
Caspase 3
Caspase 9
Cytochromes c
Ellagic Acid
Hydrolyzable Tannins
Neuroprotective Agents
Protein Kinases
Reactive Oxygen Species
Sincalide
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018; 13:757–772. PMID: 29731617.
Article2. Chung MJ, Lee S, Park YI, Lee J, Kwon KH. Neuroprotective effects of phytosterols and flavonoids from Cirsium setidens and Aster scaber in human brain neuroblastoma SK-N-SH cells. Life Sci. 2016; 148:173–182. PMID: 26874034.
Article3. Kim S, Chin YW, Cho J. Protection of cultured cortical neurons by luteolin against oxidative damage through inhibition of apoptosis and induction of heme oxygenase-1. Biol Pharm Bull. 2017; 40:256–265. PMID: 28250268.
Article4. Moslehi M, Yazdanparast R. SK-N-MC cell death occurs by distinct molecular mechanisms in response to hydrogen peroxide and superoxide anions: involvements of JAK2-STAT3, JNK, and p38 MAP kinases pathways. Cell Biochem Biophys. 2013; 66:817–829. PMID: 23417568.
Article5. Tian X, Gao L, An L, Jiang X, Bai J, Huang J, Meng W, Zhao Q. Pretreatment of MQA, a caffeoylquinic acid derivative compound, protects against H2O2-induced oxidative stress in SH-SY5Y cells. Neurol Res. 2016; 38:1079–1087. PMID: 27800716.6. Tian X, Sui S, Huang J, Bai JP, Ren TS, Zhao QC. Neuroprotective effects of Arctium lappa L. roots against glutamate-induced oxidative stress by inhibiting phosphorylation of p38, JNK and ERK 1/2 MAPKs in PC12 cells. Environ Toxicol Pharmacol. 2014; 38:189–198. PMID: 24956398.7. Gay NH, Phopin K, Suwanjang W, Songtawee N, Ruankham W, Wongchitrat P, Prachayasittikul S, Prachayasittikul V. Neuroprotective effects of phenolic and carboxylic acids on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Neurochem Res. 2018; 43:619–636. PMID: 29417471.
Article8. Nataraj J, Manivasagam T, Justin Thenmozhi A, Essa MM. Neuroprotective effect of asiatic acid on rotenone-induced mitochondrial dysfunction and oxidative stress-mediated apoptosis in differentiated SH-SYS5Y cells. Nutr Neurosci. 2017; 20:351–359. PMID: 26856988.
Article9. Chen C, Cao J, Ma X, Wang X, Chen Q, Yan S, Zhao N, Geng Z, Wang Z. Neuroprotection by polynitrogen manganese complexes: regulation of reactive oxygen species-related pathways. Sci Rep. 2016; 6:20853. PMID: 26857964.
Article10. Park HR, Lee H, Park H, Jeon JW, Cho WK, Ma JY. Neuroprotective effects of Liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. BMC Complement Altern Med. 2015; 15:171. PMID: 26054856.
Article11. DaSilva NA, Nahar PP, Ma H, Eid A, Wei Z, Meschwitz S, Zawia NH, Slitt AL, Seeram NP. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr Neurosci. 2019; 22:185–195. PMID: 28784051.12. Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013; 24:1415–1422. PMID: 23849454.
Article13. Wang Y, Qiu Z, Zhou B, Liu C, Ruan J, Yan Q, Liao J, Zhu F. In vitro antiproliferative and antioxidant effects of urolithin A, the colonic metabolite of ellagic acid, on hepatocellular carcinomas HepG2 cells. Toxicol In Vitro. 2015; 29:1107–1115. PMID: 25910917.14. Esteban-Fernández A, Rendeiro C, Spencer JP, Del Coso DG, de Llano MD, Bartolomé B, Moreno-Arribas MV. Neuroprotective effects of selected microbial-derived phenolic metabolites and aroma compounds from wine in human SH-SY5Y neuroblastoma cells and their putative mechanisms of action. Front Nutr. 2017; 4:3. PMID: 28352628.
Article15. Liu QS, Li SR, Li K, Li X, Yin X, Pang Z. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/β-catenin signaling in vivo and in vitro. Mol Nutr Food Res. 2017; 61:1600587.16. Komatsu W, Kishi H, Yagasaki K, Ohhira S. Urolithin A attenuates pro-inflammatory mediator production by suppressing PI3-K/Akt/NF-κB and JNK/AP-1 signaling pathways in lipopolysaccharide-stimulated RAW264 macrophages: possible involvement of NADPH oxidase-derived reactive oxygen species. Eur J Pharmacol. 2018; 833:411–424. PMID: 29932926.
Article17. Kang I, Kim Y, Tomás-Barberán FA, Espín JC, Chung S. Urolithin A, C, and D, but not iso-urolithin A and urolithin B, attenuate triglyceride accumulation in human cultures of adipocytes and hepatocytes. Mol Nutr Food Res. 2016; 60:1129–1138. PMID: 26872561.
Article18. Tang L, Mo Y, Li Y, Zhong Y, He S, Zhang Y, Tang Y, Fu S, Wang X, Chen A. Urolithin A alleviates myocardial ischemia/reperfusion injury via PI3K/Akt pathway. Biochem Biophys Res Commun. 2017; 486:774–780. PMID: 28343995.
Article19. Wang S, Xia B, Qiao Z, Duan L, Wang G, Meng W, Liu Z, Wang Y, Zhang M. Tetramethylpyrazine attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells through regulating apoptosis, autophagy and oxidative damage. Drug Des Devel Ther. 2019; 13:1187–1196.20. Tu G, Zhang YF, Wei W, Li L, Zhang Y, Yang J, Xing Y. Allicin attenuates H2O2-induced cytotoxicity in retinal pigmented epithelial cells by regulating the levels of reactive oxygen species. Mol Med Rep. 2016; 13:2320–2326. PMID: 26781848.21. Kim KB, Lee S, Kang I, Kim JH. Momordica charantia ethanol extract attenuates H2O2-induced cell death by its antioxidant and anti-apoptotic properties in human neuroblastoma SK-N-MC cells. Nutrients. 2018; 10:E1368. PMID: 30249986.22. Lee KH, Lee SJ, Lee HJ, Choi GE, Jung YH, Kim DI, Gabr AA, Ryu JM, Han HJ. Amyloid β1-42 (Aβ1-42) induces the CDK2-mediated phosphorylation of tau through the activation of the mTORC1 signaling pathway while promoting neuronal cell death. Front Mol Neurosci. 2017; 10:229. PMID: 28790888.
Article23. Lee HJ, Ryu JM, Jung YH, Lee SJ, Kim JY, Lee SH, Hwang IK, Seong JK, Han HJ. High glucose upregulates BACE1-mediated Aβ production through ROS-dependent HIF-1α and LXRα/ABCA1-regulated lipid raft reorganization in SK-N-MC cells. Sci Rep. 2016; 6:36746. PMID: 27829662.
Article24. Gerhauser C. Impact of dietary gut microbial metabolites on the epigenome. Philos Trans R Soc Lond B Biol Sci. 2018; 373:20170359. PMID: 29685968.
Article25. Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med (Encinitas). 2014; 13:35–43.26. Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008; 269:262–268. PMID: 18468784.
Article27. Espín JC, Larrosa M, García-Conesa MT, Tomás-Barberán F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med. 2013; 2013:270418. PMID: 23781257.
Article28. Kojadinovic M, Arsic A, Petovic-Oggiano G, Gavrovic-Jankulovic M, Glibetic M, Popovic M. Effect of urolithins on oxidative stress of colorectal adenocarcinomacells-Caco-2. Int J Food Sci Nutr. 2017; 68:952–959. PMID: 28535698.
Article29. Zhao W, Shi F, Guo Z, Zhao J, Song X, Yang H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol Carcinog. 2018; 57:193–200. PMID: 28976622.
Article30. Liberal J, Carmo A, Gomes C, Cruz MT, Batista MT. Urolithins impair cell proliferation, arrest the cell cycle and induce apoptosis in UMUC3 bladder cancer cells. Invest New Drugs. 2017; 35:671–681. PMID: 28631098.
Article31. González-Sarrías A, García-Villalba R, Núñez-Sánchez MA, Tomé-Carneiro J, Zafrilla P, Mulero J, Tomás-Barberán FA, Espín JC. Identifying the limits for ellagic acid bioavailability: a crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J Funct Foods. 2015; 19:225–235.
Article32. Nuñez-Sánchez MA, García-Villalba R, Monedero-Saiz T, García-Talavera NV, Gómez-Sánchez MB, Sánchez-Álvarez C, García-Albert AM, Rodríguez-Gil FJ, Ruiz-Marín M, Pastor-Quirante FA, Martínez-Díaz F, Yáñez-Gascón MJ, González-Sarrías A, Tomás-Barberán FA, Espín JC. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res. 2014; 58:1199–1211. PMID: 24532260.
Article33. Cerdá B, Espín JC, Parra S, Martínez P, Tomás-Barberán FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004; 43:205–220. PMID: 15309440.
Article34. Vicinanza R, Zhang Y, Henning SM, Heber D. Pomegranate juice metabolites, ellagic acid and urolithin A, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis. Evid Based Complement Alternat Med. 2013; 2013:247504. PMID: 23710216.
Article35. Kujawska M, Jodynis-Liebert J. Polyphenols in Parkinson's disease: a systematic review of in vivo studies. Nutrients. 2018; 10:E642. PMID: 29783725.36. Figueira I, Garcia G, Pimpão RC, Terrasso AP, Costa I, Almeida AF, Tavares L, Pais TF, Pinto P, Ventura MR, Filipe A, McDougall GJ, Stewart D, Kim KS, Palmela I, Brites D, Brito MA, Brito C, Santos CN. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci Rep. 2017; 7:11456. PMID: 28904352.
Article37. Gasperotti M, Passamonti S, Tramer F, Masuero D, Guella G, Mattivi F, Vrhovsek U. Fate of microbial metabolites of dietary polyphenols in rats: is the brain their target destination? ACS Chem Neurosci. 2015; 6:1341–1352. PMID: 25891864.
Article38. Saha P, Yeoh BS, Singh R, Chandrasekar B, Vemula PK, Haribabu B, Vijay-Kumar M, Jala VR. Gut microbiota conversion of dietary ellagic acid into bioactive phytoceutical urolithin A inhibits heme peroxidases. PLoS One. 2016; 11:e0156811. PMID: 27254317.
Article39. Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Félix AA, Williams EG, Jha P, Lo Sasso G, Huzard D, Aebischer P, Sandi C, Rinsch C, Auwerx J. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016; 22:879–888. PMID: 27400265.40. Choi DJ, Cho S, Seo JY, Lee HB, Park YI. Neuroprotective effects of the Phellinus linteus ethyl acetate extract against H2O2-induced apoptotic cell death of SK-N-MC cells. Nutr Res. 2016; 36:31–43. PMID: 26773779.41. Ishimoto H, Tai A, Yoshimura M, Amakura Y, Yoshida T, Hatano T, Ito H. Antioxidative properties of functional polyphenols and their metabolites assessed by an ORAC assay. Biosci Biotechnol Biochem. 2012; 76:395–399. PMID: 22313776.
Article42. Sun S, Pan S, Miao A, Ling C, Pang S, Tang J, Chen D, Zhao C. Active extracts of black tea (Camellia sinensis) induce apoptosis of PC-3 prostate cancer cells via mitochondrial dysfunction. Oncol Rep. 2013; 30:763–772. PMID: 23715786.43. Chen XZ, Li JN, Zhang YQ, Cao ZY, Liu ZZ, Wang SQ, Liao LM, Du J. Fuzheng Qingjie recipe induces apoptosis in HepG2 cells via P38 MAPK activation and the mitochondria-dependent apoptotic pathway. Mol Med Rep. 2014; 9:2381–2387. PMID: 24737008.
Article44. Firdaus F, Zafeer MF, Waseem M, Anis E, Hossain MM, Afzal M. Ellagic acid mitigates arsenic-trioxide-induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. J Biochem Mol Toxicol. 2018; 32:e22024.
Article45. Chen P, Chen F, Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018; 8:1465. PMID: 29362375.
Article46. Baluchnejadmojarad T, Rabiee N, Zabihnejad S, Roghani M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson's disease: possible involvement of ERβ/Nrf2/HO-1 signaling. Brain Res. 2017; 1662:23–30. PMID: 28238669.
Article47. Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996; 27:1124–1129. PMID: 8650725.
Article48. Li J, O W, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013; 14:24438–24475. PMID: 24351827.
Article49. Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010; 1802:396–405. PMID: 20079433.
Article50. Chen SY, Zheng K, Wang Z. Neuroprotective effects of ellagic acid on neonatal hypoxic brain injury via inhibition of inflammatory mediators and down-regulation of JNK/p38 MAPK activation. Trop J Pharm Res. 2016; 15:241–251.
Article51. González-Sarrías A, Larrosa M, Tomás-Barberán FA, Dolara P, Espín JC. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr. 2010; 104:503–512. PMID: 20338073.
Article52. Munoz L, Ralay Ranaivo H, Roy SM, Hu W, Craft JM, McNamara LK, Chico LW, Van Eldik LJ, Watterson DM. A novel p38 alpha MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer's disease mouse model. J Neuroinflammation. 2007; 4:21. PMID: 17784957.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective effects of Momordica charantia extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SK-N-MC cells
- Inhibition of Store-Operated Calcium Entry Protects Endothelial Progenitor Cells from Hâ‚‚Oâ‚‚-Induced Apoptosis
- Acer okamotoanum Inhibit the Hydrogen Peroxide-Induced Oxidative Stress in C6 Glial Cells
- Propofol protects against oxidative-stress-induced COS-7 cell apoptosis by inducing autophagy
- Protective effects of perilla oil and alpha linolenic acid on SH-SY5Y neuronal cell death induced by hydrogen peroxide