Nat Prod Sci.
2018 Sep;24(3):148-154. 10.20307/nps.2018.24.3.148.
Acer okamotoanum Inhibit the Hydrogen Peroxide-Induced Oxidative Stress in C6 Glial Cells
- Affiliations
-
- 1Department of Food Science and Nutrition & Kimchi Research Institute, Pusan National University, Busan 46241, Republic of Korea. ejcho@pusan.ac.kr
- 2Department of Integrative Plant Science, Chung-Ang University, Anseong 17546, Republic of Korea. slee@cau.ac.kr
- KMID: 2422005
- DOI: http://doi.org/10.20307/nps.2018.24.3.148
Abstract
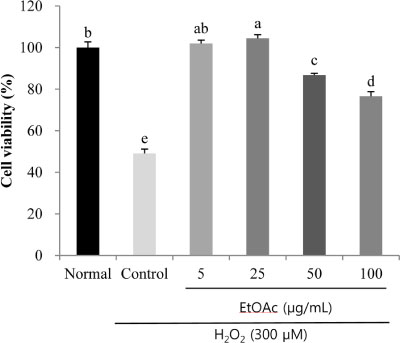
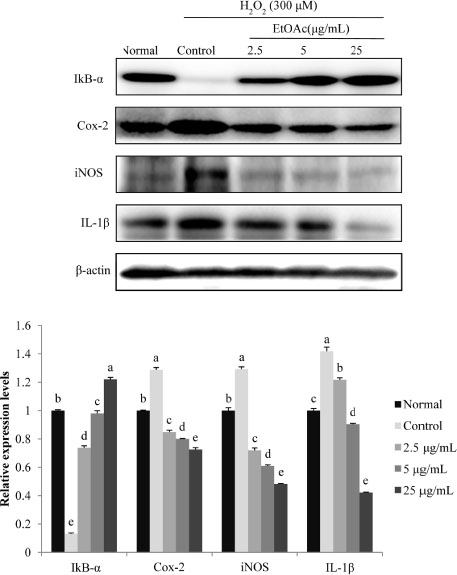
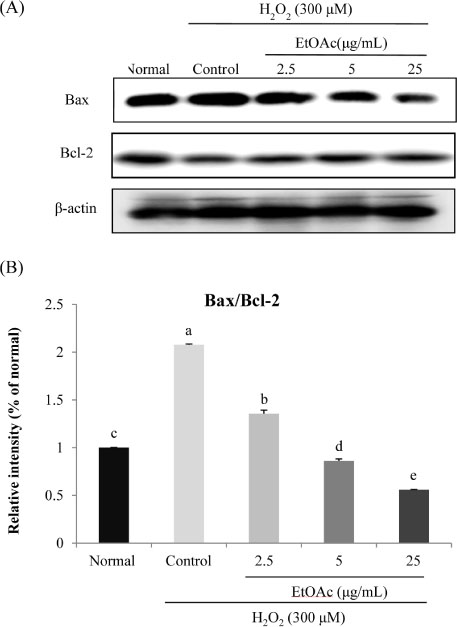
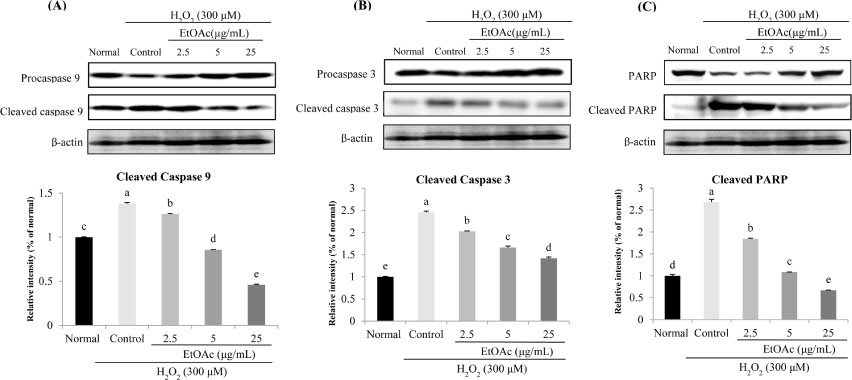
- Chronic oxidative stress due to the accumulation of reactive oxygen species (ROS) in neuronal cells ultimately leads to neurodegenerative diseases. The use of natural therapies for the prevention of ROS-induced cell damage and for the treatment of neurodegenerative disorders has shown promising results. In this study, we evaluated the neuroprotective effects of the ethyl acetate (EtOAc) fraction of A. Okamotoanum against the hydrogen peroxide (H₂O₂)-induced oxidative stress in C6 glial cells. Results show that cell viability was decreased in cells incubated with H₂O₂, whereas the addition of EtOAc fraction treatments in such cells significantly increased viability. The EtOAc fraction showed the highest inhibitory activity against ROS production and it also decreased the expressions of inflammatory proteins including cyclooxygenase-2, inducible nitric oxide synthase and interleukin-1β. Furthermore, the EtOAc fraction inhibited apoptosis by regulating the protein expressions cleaved caspase -9, -3, poly ADP ribose polymerase, Bax and Bcl-2. Therefore, these results show that the EtOAc fraction of A. Okamotoanum exhibits neuroprotective effects against H₂O₂ induced oxidative damage by regulating the inflammatory reaction and apoptotic pathway.
MeSH Terms
-
Acer*
Apoptosis
Cell Survival
Cyclooxygenase 2
Hydrogen Peroxide
Hydrogen*
Inflammation
Neurodegenerative Diseases
Neuroglia*
Neurons
Neuroprotective Agents
Nitric Oxide Synthase Type II
Oxidative Stress*
Poly(ADP-ribose) Polymerases
Reactive Oxygen Species
Cyclooxygenase 2
Hydrogen
Hydrogen Peroxide
Neuroprotective Agents
Nitric Oxide Synthase Type II
Poly(ADP-ribose) Polymerases
Reactive Oxygen Species
Figure
Reference
-
1. Heo SJ, Jeon YJ. J Appl Phycol. 2008; 20:1087–1095.2. Ko SC, Kim D, Jeon Y. J Food Chem Toxicol. 2012; 50:2294–2302.3. Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA. Biomed Pharmacother. 2015; 74:101–110.4. Qin B, Panickar KS, Anderson RA. Life Sci. 2014; 102:72–79.5. Kim HJ, Woo ER, Shin CG, Park HJ. Nat Prod. 1998; 61:145–148.6. Jin W, Thuong PT, Su ND, Min BS, Son KH, Chang HW, Kim HP, Kang SS, Sok DE, Bae K. Arch Pharm Res. 2007; 30:275–281.7. Jeong MH, Ha JH, Oh SH, Kim SS, Lee HJ, Kang HY, Lee HY. Korean J Med Crop Sci. 2009; 17:407–411.8. Jeong MH, Kim SS, Kim JS, Lee HJ, Choi GP, Lee HY. J Korean For Soc. 2010; 99:470–478.9. Jin L, Han JG, Ha JH, Jeong HS, Kwon MC, Jeong MH, Lee HJ, Kang HY, Choi DH, Lee HY. Korean J Med Crop Sci. 2008; 16:427–433.10. Qadir SA, Kim CH, Kwon MC, Lee HJ, Kang HY, Choi DH, Lee HY. Korean J Med Crop Sci. 2007; 15:405–410.11. Choi SY, Kim JH, Lee JM, Lee SH, Cho EJ. J Appl Biol Chem. 2017; 60:141–147.12. Mosmann T. J Immunol Methods. 1983; 65:55–63.13. Ma WW, Hou CC, Zhou X, Yu HL, Xi YD, Ding J, Zhao X, Xiao R. Neurochem Res. 2013; 38:1315–1323.14. Park JH, Kim CK, Lee SB, Lee KH, Cho SW, Ahn JY. Sci Rep. 2016; 6:21857.15. Park HR, Lee H, Park H, Jeon JW, Cho WK, Ma JY. BMC Complement Altern Med. 2015; 15:171–181.16. Kwon SH, Hong SI, Ma SX, Lee SY, Jang CG. Food Chem Toxicol. 2015; 80:41–51.17. Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Psychopharmacology. 2015; 232:931–942.18. Barnham KJ, Masters CL, Bush AI. Nat Rev Drug Discov. 2004; 3:205–214.19. Malek R, Borowicz KK, Jargiello M, Czuczwar SJ. Pharmacol Rep. 2007; 59:25–33.20. Janssen-Heininger YM, Poynter ME, Baeuerle PA. Free Radic Biol Med. 2000; 28:1317–1327.21. Moneada S, Palmer RM, Higgs EA. Pharmacol Rev. 1991; 43:109–142.22. Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, Xie QW, Nathan C, Gribble GW, Sporn MB. Cancer Res. 1998; 58:717–723.23. DuBois RN, Radhika A, Reddy BS, Entingh AJ. Gastroenterology. 1996; 110:1259–1262.24. Ogura M, Takarada T, Nakamichi N, Kawagoe H, Sako A, Nakazato R, Yoneda Y. Neurochem Int. 2011; 58:504–511.25. Quincozes-Santos A, Bobermin LD, Latini A, Wajner M, Souza DO, Gonçalves CA, Gottfried C. PLoS One. 2013; 8:e64372.26. Pahl HL. Oncogene. 1999; 18:6853–6866.27. Shi X, Dong Z, Huang C, Ma W, Liu K, Ye J, Chen F, Leonard SS, Ding M, Castranova V, Vallyathan V. Mol Cell Biochem. 1999; 194:63–70.
Article28. Choi SY, Lee J, Lee DG, Lee S, Cho EJ. Appl Biol Chem. 2017; 60:1–9.29. Alamdary SZ, Khodagholi F, Shaerzadeh F, Ansari N, Sonboli A, Tusi SK. Cytotechnology. 2012; 64:403–419.30. Kitamura Y, Shimohama S, Kamoshima W, Ota T, Matsuoka Y, Nomura Y, Smith MA, Perry G, Whitehouse PJ, Taniguchi T. Brain Res. 1998; 780:260–269.31. Ly JD, Grubb DR, Lawen A. Apoptosis. 2003; 8:115–128.
Article32. Kwon SH, Kim MJ, Ma SX, You IJ, Hwang JY, Oh JH, Kim SY, Kim HC, Lee SY, Jang CG. J Ethnopharmacol. 2012; 142:337–345.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- The Neuro-Protective Effect of the Methanolic Extract of Perilla frutescens var. japonica and Rosmarinic Acid against H2O2-Induced Oxidative Stress in C6 Glial Cells
- Role of Morphine in the Glutamate-Induced Oxidative Damage of C6 Glial Cells
- Protective effect of Cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro
- Role of Ascorbic Acid Against the Oxidative Stress in Trabecular Meshwork Cells