J Dent Anesth Pain Med.
2017 Mar;17(1):37-46. 10.17245/jdapm.2017.17.1.37.
Propofol protects against oxidative-stress-induced COS-7 cell apoptosis by inducing autophagy
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, School of Dentistry, Pusan National University, Dental Research Institute, Yangsan, Republic of Korea. chemfrie@naver.com
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan, Republic of Korea.
- 3Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Republic of Korea.
- KMID: 2374722
- DOI: http://doi.org/10.17245/jdapm.2017.17.1.37
Abstract
- BACKGROUND
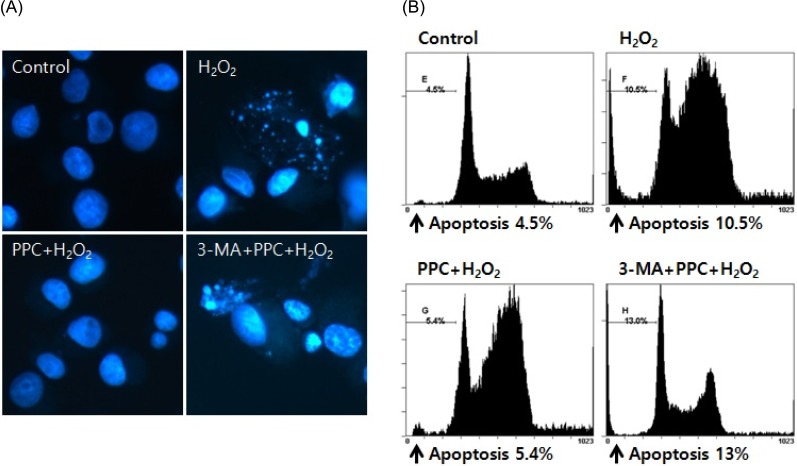
In oxidative stress, reactive oxygen species (ROS) production contributes to cellular dysfunction and initiates the apoptotic cascade. Autophagy is considered the mechanism that decreases ROS concentration and oxidative damage. Propofol shows antioxidant properties, but the mechanisms underlying the effect of propofol preconditioning (PPC) on oxidative injury remain unclear. Therefore, we investigated whether PPC protects against cell damage from hydrogen peroxide (Hâ‚‚Oâ‚‚)-induced oxidative stress and influences cellular autophagy. METHOD: COS-7 cells were randomly divided into the following groups: control, cells were incubated in normoxia (5% COâ‚‚, 21% Oâ‚‚, and 74% Nâ‚‚) for 24 h without propofol; Hâ‚‚Oâ‚‚, cells were exposed to Hâ‚‚Oâ‚‚ (400 µM) for 2 h; PPC + Hâ‚‚Oâ‚‚, cells pretreated with propofol were exposed to Hâ‚‚Oâ‚‚; and 3-methyladenine (3-MA) + PPC + Hâ‚‚Oâ‚‚, cells pretreated with 3-MA (1 mM) for 1 h and propofol were exposed to Hâ‚‚Oâ‚‚. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide thiazolyl blue (MTT) reduction. Apoptosis was determined using Hoechst 33342 staining and fluorescence microscopy. The relationship between PPC and autophagy was detected using western blot analysis.
RESULTS
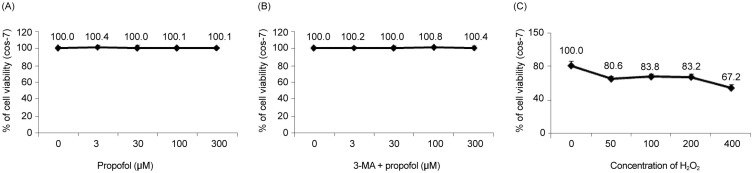
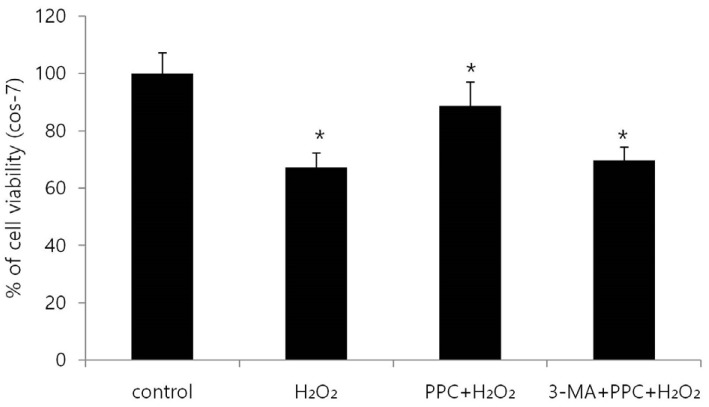
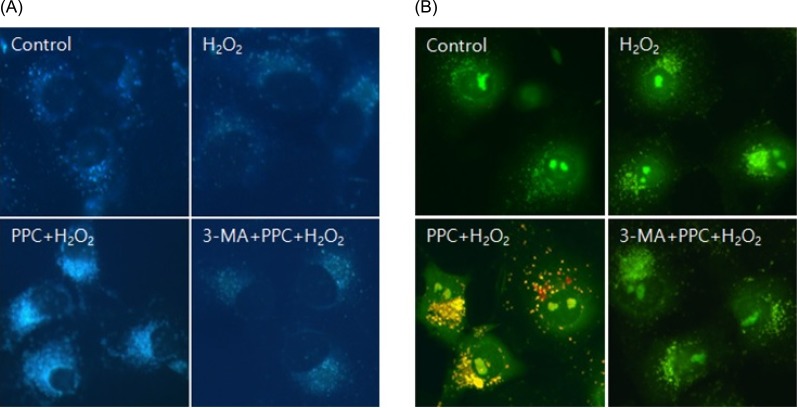
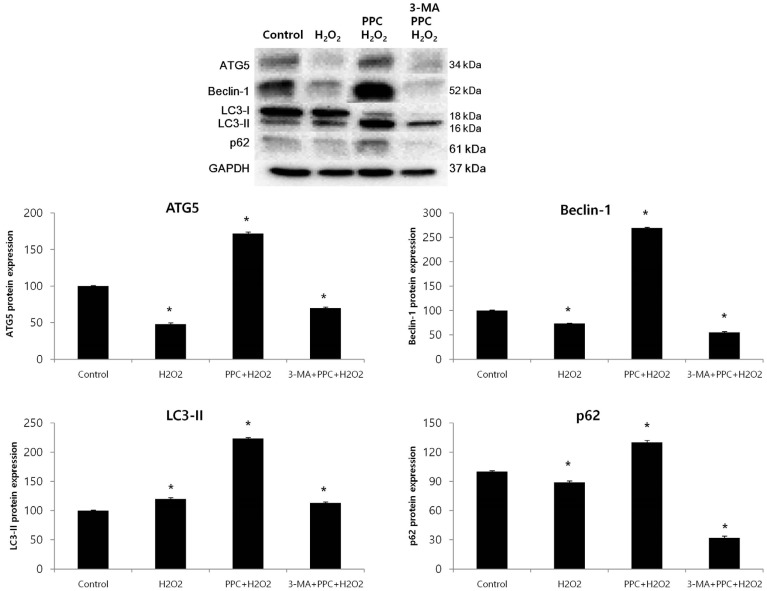
Cell viability decreased more significantly in the Hâ‚‚Oâ‚‚ group than in the control group, but it was improved by PPC (100 µM). Pretreatment with propofol effectively decreased Hâ‚‚Oâ‚‚-induced COS-7 cell apoptosis. However, pretreatment with 3-MA inhibited the protective effect of propofol during apoptosis. Western blot analysis showed that the level of autophagy-related proteins was higher in the PPC + Hâ‚‚Oâ‚‚ group than that in the H2O2 group.
CONCLUSION
PPC has a protective effect on Hâ‚‚Oâ‚‚-induced COS-7 cell apoptosis, which is mediated by autophagy activation.
Keyword
MeSH Terms
Figure
Reference
-
1. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408:239–247. PMID: 11089981.
Article2. Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000; 97:8010–8014. PMID: 10869423.
Article3. Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. general properties and effect of hyperbaric oxygen. Biochem J. 1973; 134:707–716. PMID: 4749271.
Article4. Chien CT, Lee PH, Chen CF, Ma MC, Lai MK, Hsu SM. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J Am Soc Nephrol. 2001; 12:973–982. PMID: 11316856.5. Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015; 22:377–388. PMID: 25257172.
Article6. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010; 40:280–293. PMID: 20965422.
Article7. Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007; 14:146–157. PMID: 16645637.
Article8. Filomeni G, Desideri E, Cardaci S, Rotilio G, Ciriolo MR. Under the ROS...thiol network is the principal suspect for autophagy commitment. Autophagy. 2010; 6:999–1005. PMID: 20639698.9. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417:1–13. PMID: 19061483.
Article10. Tesauro M, Thompson WC, Moss J. Effect of staurosporine-induced apoptosis on endothelial nitric oxide synthase in transfected COS-7 cells and primary endothelial cells. Cell Death Differ. 2006; 13:597–606. PMID: 16195740.
Article11. Xu JJ, Wang YL. Propofol attenuation of hydrogen peroxide-mediated oxidative stress and apoptosis in cultured cardiomyocytes involves haeme oxygenase-1. Eur J Anaesthesiol. 2008; 25:395–402. PMID: 18289444.
Article12. Kobayashi K, Yoshino F, Takahashi SS, Todoki K, Maehata Y, Komatsu T, et al. Direct assessments of the antioxidant effects of propofol medium chain triglyceride/long chain triglyceride on the brain of stroke-prone spontaneously hypertensive rats using electron spin resonance spectroscopy. Anesthesiology. 2008; 109:426–435. PMID: 18719440.
Article13. Ansley DM, Lee J, Godin DV, Garnett ME, Qayumi AK. Propofol enhances red cell antioxidant capacity in swine and humans. Can J Anaesth. 1998; 45:233–239. PMID: 9579261.
Article14. Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. Volatile anesthetic-induced cardiac preconditioning. J Anesth. 2007; 21:212–219. PMID: 17458651.
Article15. Liu KX, Rinne T, He W, Wang F, Xia Z. Propofol attenuates intestinal mucosa injury induced by intestinal ischemia-reperfusion in the rat. Can J Anaesth. 2007; 54:366–374. PMID: 17470888.
Article16. Kamada N, Kanaya N, Hirata N, Kimura S, Namiki A. Cardioprotective effects of propofol in isolated ischemia-reperfused guinea pig hearts: Role of KATP channels and GSK-3beta. Can J Anaesth. 2008; 55:595–605. PMID: 18840589.17. Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. part I. signalling and cytoprotective mechanisms. Br J Anaesthz. 2003; 91:551–565.
Article18. Das M, Das DK. Molecular mechanism of preconditioning. IUBMB Life. 2008; 60:199–203. PMID: 18344203.
Article19. Assad AR, Delou JM, Fonseca LM, Villela NR, Nascimento JH, Vercosa N, et al. The role of KATP channels on propofol preconditioning in a cellular model of renal ischemia-reperfusion. Anesth Analg. 2009; 109:1486–1492. PMID: 19843786.
Article20. Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988; 240:1302–1309. PMID: 3287616.
Article21. Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007; 292:C670–C686. PMID: 17020935.
Article22. Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003; 17:1195–1214. PMID: 12832285.
Article23. Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, et al. Hydroxyl radicals and DNA base damage. Mutat Res. 1999; 424:9–21. PMID: 10064846.
Article24. Wang H, Xue Z, Wang Q, Feng X, Shen Z. Propofol protects hepatic L02 cells from hydrogen peroxide-induced apoptosis via activation of extracellular signal-regulated kinases pathway. Anesth Analg. 2008; 107:534–540. PMID: 18633031.
Article25. Wu XJ, Zheng YJ, Cui YY, Zhu L, Lu Y, Chen HZ. Propofol attenuates oxidative stress-induced PC12 cell injury via p38 MAP kinase dependent pathway. Acta Pharmacol Sin. 2007; 28:1123–1128. PMID: 17640472.
Article26. Li Volti G, Basile F, Murabito P, Galvano F, Di Giacomo C, Gazzolo D, et al. Antioxidant properties of anesthetics: The biochemist, the surgeon and the anesthetist. Clin Ter. 2008; 159:463–469. PMID: 19169610.27. Li Volti G, Sorrenti V, Murabito P, Galvano F, Veroux M, Gullo A, et al. Pharmacological induction of heme oxygenase-1 inhibits iNOS and oxidative stress in renal ischemia-reperfusion injury. Transplant Proc. 2007; 39:2986–2991. PMID: 18089306.
Article28. Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: A novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002; 61:554–561. PMID: 11854435.
Article29. Acquaviva R, Campisi A, Murabito P, Raciti G, Avola R, Mangiameli S, et al. Propofol attenuates peroxynitrite-mediated DNA damage and apoptosis in cultured astrocytes: An alternative protective mechanism. Anesthesiology. 2004; 101:1363–1371. PMID: 15564944.30. Hayashi K, Dan K, Goto F, Tshuchihashi N, Nomura Y, Fujioka M, et al. The autophagy pathway maintained signaling crosstalk with the Keap1-Nrf2 system through p62 in auditory cells under oxidative stress. Cell Signal. 2015; 27:382–393. PMID: 25435427.
Article31. Huang Q, Wu YT, Tan HL, Ong CN, Shen HM. A novel function of poly(ADP-ribose) polymerase-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Differ. 2009; 16:264–277. PMID: 18974775.
Article32. Huang Q, Shen HM. To die or to live: The dual role of poly(ADP-ribose) polymerase-1 in autophagy and necrosis under oxidative stress and DNA damage. Autophagy. 2009; 5:273–276. PMID: 19139632.
Article33. Glick D, Barth S, Macleod KF. Autophagy: Cellular and molecular mechanisms. J Pathol. 2010; 221:3–12. PMID: 20225336.
Article34. Mori F, Tanji K, Odagiri S, Toyoshima Y, Yoshida M, Kakita A, et al. Autophagy-related proteins (p62, NBR1 and LC3) in intranuclear inclusions in neurodegenerative diseases. Neurosci Lett. 2012; 522:134–138. PMID: 22728060.
Article35. Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004; 432:1032–1036. PMID: 15525940.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Remifentanil induces autophagy and prevents hydrogen peroxide-induced apoptosis in Cos-7 cells
- Propofol protects human keratinocytes from oxidative stress via autophagy expression
- Effects of propofol-induced autophagy against oxidative stress in human osteoblasts
- Propofol Attenuates Hypoxia/Reoxygenation-Induced Apoptosis and Autophagy in HK-2 Cells by Inhibiting JNK Activation
- Role of Apigenin in Cancer Prevention via the Induction of Apoptosis and Autophagy