Nutr Res Pract.
2018 Apr;12(2):93-100. 10.4162/nrp.2018.12.2.93.
Protective effects of perilla oil and alpha linolenic acid on SH-SY5Y neuronal cell death induced by hydrogen peroxide
- Affiliations
-
- 1Department of Food Science and Nutrition & Kimchi Research Institute, Pusan National University, Busandaehak-ro 63 beon-gil 2, Geumjeong-gu, Busan 46241, Korea. ejcho@pusan.ac.kr
- 2Department of Southern Area Crop Science, National Institute of Crop Science, Rural Development Administration, Gyeongnam 50424, Korea.
- 3Department of Integrative Plant Science, Chung-Ang University, Anseong 17546, Korea.
- KMID: 2407805
- DOI: http://doi.org/10.4162/nrp.2018.12.2.93
Abstract
- BACKGROUND
/OBJECTIVE: Oxidative stress plays a key role in neuronal cell damage, which is associated with neurodegenerative disease. The aim of present study was to investigate the neuroprotective effects of perilla oil (PO) and its active component, alpha-linolenic acid (ALA), against hydrogen peroxide (Hâ‚‚Oâ‚‚)-induced oxidative stress in SH-SY5Y neuronal cells.
MATERIALS/METHODS
The SH-SY5Y human neuroblastoma cells exposed to 250 µM Hâ‚‚Oâ‚‚ for 24 h were treated with different concentrations of PO (25, 125, 250 and 500 µg/mL) and its major fatty acid, ALA (1, 2.5, 5 and 25 µ/mL). We examined the effects of PO and ALA on Hâ‚‚Oâ‚‚-induced cell viability, lactate dehydrogenase (LDH) release, and nuclear condensation. Moreover, we determined whether PO and ALA regulated the apoptosis-related protein expressions, such as cleaved-poly ADP ribose polymerase (PARP), cleaved caspase-9 and -3, BCL-2 and BAX.
RESULTS
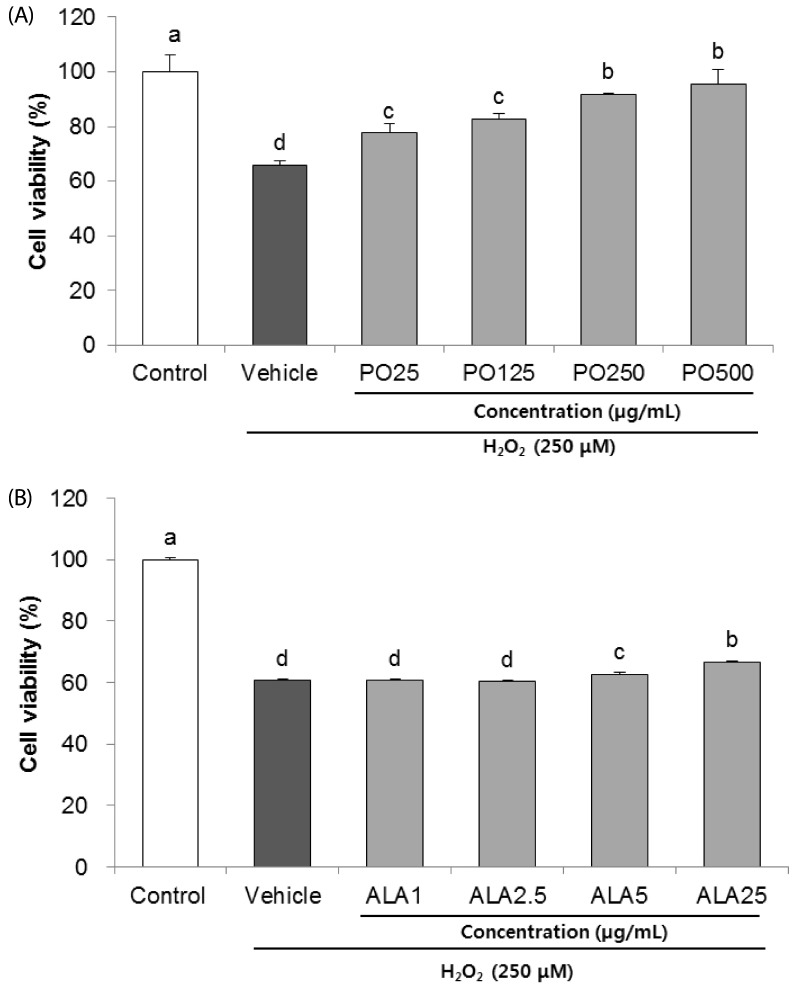
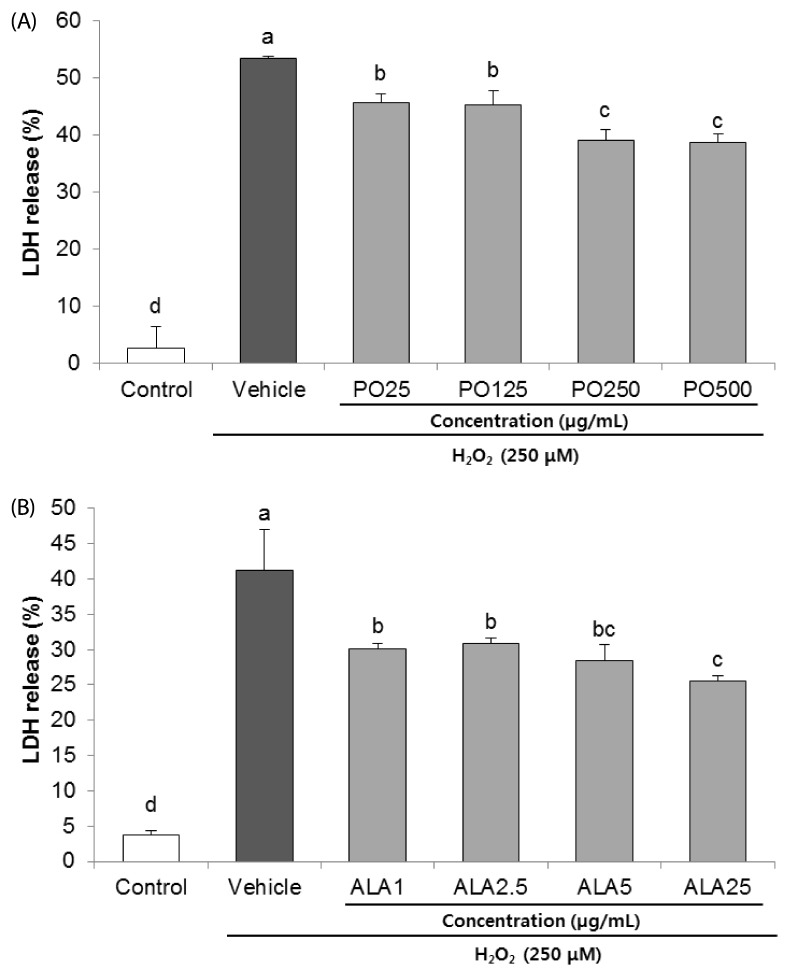
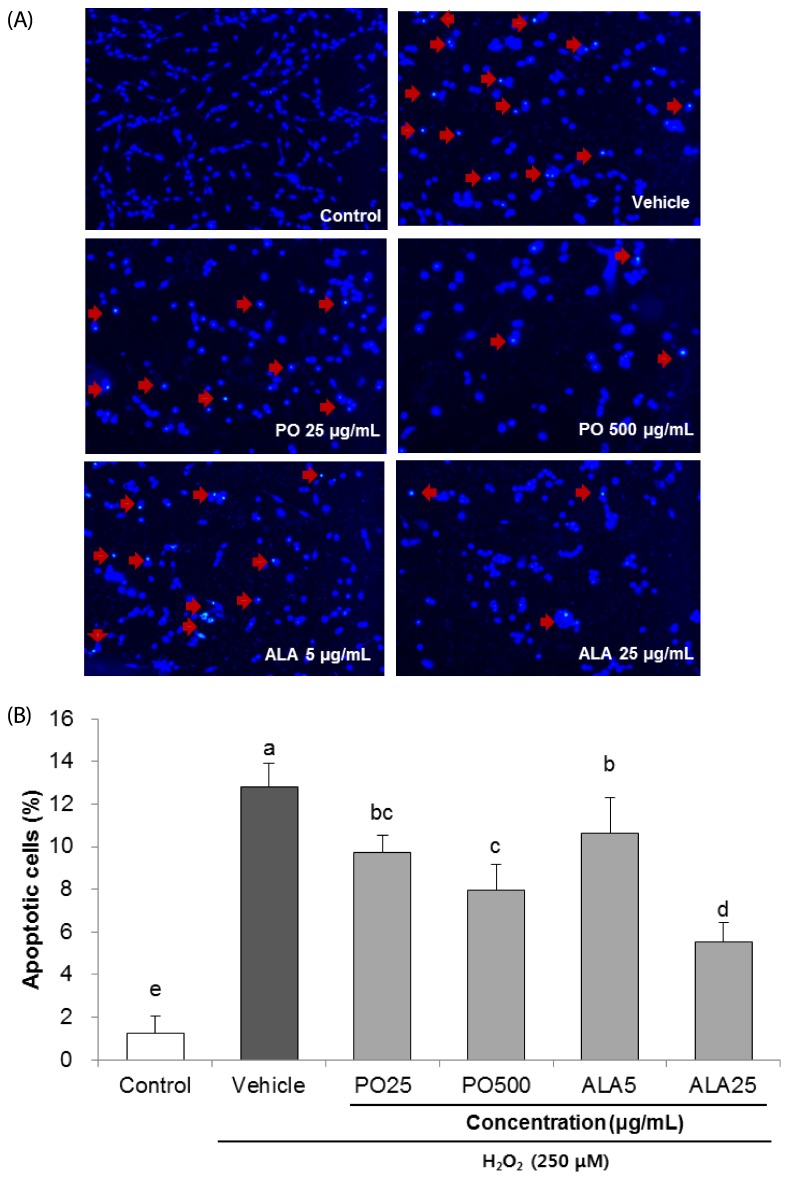
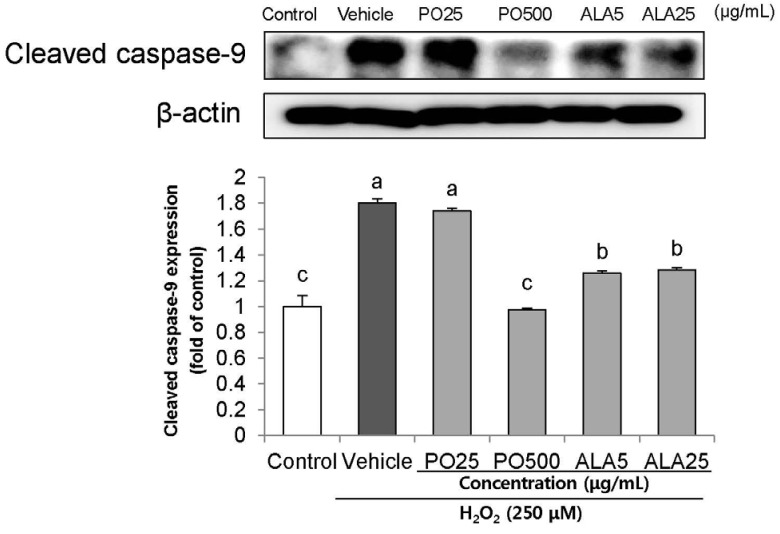
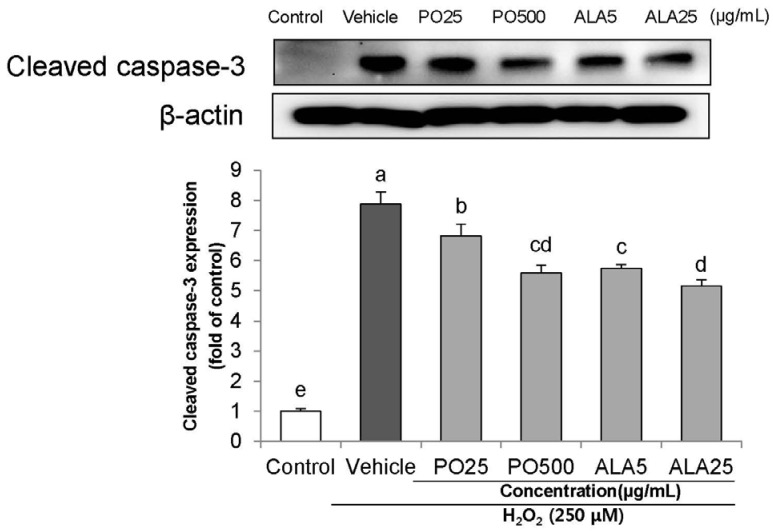
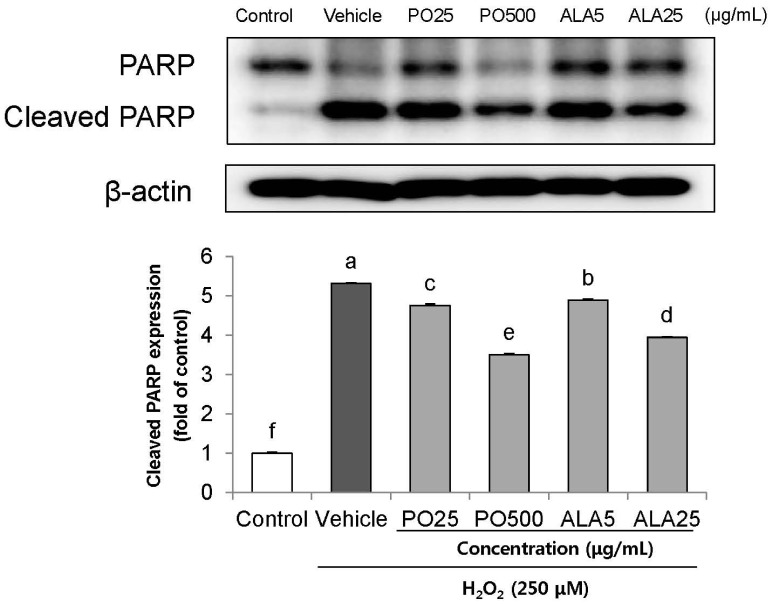
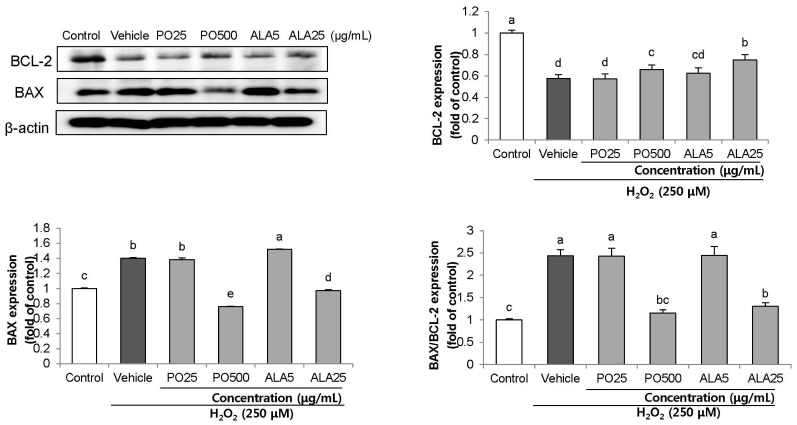
Treatment of Hâ‚‚Oâ‚‚ resulted in decreased cell viability, increased LDH release, and increase in the nuclei condensation as indicated by Hoechst 33342 staining. However, PO and ALA treatment significantly attenuated the neuronal cell death, indicating that PO and ALA potently blocked the Hâ‚‚Oâ‚‚-induced neuronal apoptosis. Furthermore, cleaved-PARP, cleaved caspase-9 and -3 activations were significantly decreased in the presence of PO and ALA, and the Hâ‚‚Oâ‚‚-mediated up-regulated BAX/BCL-2 ratio was blocked after treatment with PO and ALA.
CONCLUSIONS
PO and its main fatty acid, ALA, exerted the protective activity from neuronal oxidative stress induced by Hâ‚‚Oâ‚‚. They regulated apoptotic pathway in neuronal cell death by alleviation of BAX/BCL-2 ratio, and down-regulation of cleaved-PARP and cleaved caspase-9 and -3. Although further studies are required to verify the protective mechanisms of PO and ALA from neuronal damage, PO and ALA are the promising agent against oxidative stress-induced apoptotic neuronal cell death.
MeSH Terms
-
Adenosine Diphosphate Ribose
alpha-Linolenic Acid*
Apoptosis
Caspase 9
Cell Death*
Cell Survival
Down-Regulation
Humans
Hydrogen Peroxide*
Hydrogen*
L-Lactate Dehydrogenase
Neuroblastoma
Neurodegenerative Diseases
Neurons*
Neuroprotective Agents
Oxidative Stress
Perilla*
Adenosine Diphosphate Ribose
Caspase 9
Hydrogen
Hydrogen Peroxide
L-Lactate Dehydrogenase
Neuroprotective Agents
alpha-Linolenic Acid
Figure
Cited by 1 articles
-
Effect of vegetable oils with different fatty acid composition on high-fat diet-induced obesity and colon inflammation
Shalom Sara Thomas, Youn-Soo Cha, Kyung-Ah Kim
Nutr Res Pract. 2020;14(5):425-437. doi: 10.4162/nrp.2020.14.5.425.
Reference
-
1. Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996; 21:83–86. PMID: 8882579.
Article2. Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991; 281:9–19. PMID: 1849843.
Article3. Braughler JM, Hall ED. Central nervous system trauma and stroke. I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic Biol Med. 1989; 6:289–301. PMID: 2663662.4. Moreira PI, Honda K, Liu Q, Santos MS, Oliveira CR, Aliev G, Nunomura A, Zhu X, Smith MA, Perry G. Oxidative stress: the old enemy in Alzheimer's disease pathophysiology. Curr Alzheimer Res. 2005; 2:403–408. PMID: 16248845.
Article5. Xue S, Jia L, Jia J. Hypoxia and reoxygenation increased BACE1 mRNA and protein levels in human neuroblastoma SH-SY5Y cells. Neurosci Lett. 2006; 405:231–235. PMID: 16901640.
Article6. Zheng L, Roberg K, Jerhammar F, Marcusson J, Terman A. Autophagy of amyloid beta-protein in differentiated neuroblastoma cells exposed to oxidative stress. Neurosci Lett. 2006; 394:184–189. PMID: 16297550.
Article7. Yadav NS, Wierzbicki A, Aegerter M, Caster CS, Pérez-Grau L, Kinney AJ, Hitz WD, Booth JR Jr, Schweiger B, Stecca KL, Allen SM, Blackwell M, Reiter RS, Carlson TJ, Russell SH, Feldmann KA, Pierce J, Browse J. Cloning of higher plant omega-3 fatty acid desaturases. Plant Physiol. 1993; 103:467–476. PMID: 8029334.
Article8. Oliver MF. It is more important to increase the intake of unsaturated fats than to decrease the intake of saturated fats: evidence from clinical trials relating to ischemic heart disease. Am J Clin Nutr. 1997; 66:980S–986S. PMID: 9322577.
Article9. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83:1505S–1519S. PMID: 16841861.
Article10. Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007; 43:658–677. PMID: 17664130.
Article11. Lee AY, Choi JM, Lee J, Lee MH, Lee S, Cho EJ. Effects of vegetable oils with different fatty acid compositions on cognition and memory ability in Aβ25–35-induced Alzheimer's disease mouse model. J Med Food. 2016; 19:912–921.12. Lee AY, Lee MH, Lee S, Cho EJ. Alpha-linolenic acid from Perilla frutescens var. japonica oil protects Aβ-induced cognitive impairment through regulation of APP processing and Aβ degradation. J Agric Food Chem. 2017; 65:10719–10729. PMID: 29092397.13. Skowyra M, Falguera V, Azman NA, Segovia F, Almajano MP. The effect of perilla frutescens extract on the oxidative stability of model food emulsions. Antioxidants (Basel). 2014; 3:38–54. PMID: 26784662.14. Hassan A, Ibrahim A, Mbodji K, Coëffier M, Ziegler F, Bounoure F, Chardigny JM, Skiba M, Savoye G, Déchelotte P, Marion-Letellier R. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. J Nutr. 2010; 140:1714–1721. PMID: 20724486.
Article15. Seong J, Song YO. Perilla oil rich in α-linolenic acid inhibits neuronal apoptosis and the expression of inflammation-mediator protein in apoE KO mice. Biocatal Agric Biotechnol. 2012; 1:167–173.
Article16. Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992; 32:804–812. PMID: 1471873.
Article17. Lee J, Rodriguez JP, Kim YJ, Lee MH, Cho EJ, Lee S. Fatty acid content in perilla cultivars commercial oils determined by GC analysis. Nat Prod Sci. 2016; 22:259–262.18. Lee J, Lee MH, Cho EJ, Lee S. High-yield methods for purification of α-linolenic acid from Perilla frutescens var. japonica oil. Appl Biol Chem. 2016; 59:89–94.19. Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991; 3:207–212. PMID: 1867954.
Article20. Racher AJ, Looby D, Griffiths JB. Use of lactate dehydrogenase release to assess changes in culture viability. Cytotechnology. 1990; 3:301–307. PMID: 1366664.
Article21. Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2001; 98:247–252. PMID: 11134526.
Article22. Arias E, Gallego-Sandín S, Villarroya M, García AG, López MG. Unequal neuroprotection afforded by the acetylcholinesterase inhibitors galantamine, donepezil, and rivastigmine in SH-SY5Y neuroblastoma cells: role of nicotinic receptors. J Pharmacol Exp Ther. 2005; 315:1346–1353. PMID: 16144975.
Article23. Lopes JP, Oliveira CR, Agostinho P. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-beta and prion peptides: implications for Alzheimer's disease and prion-related encephalopathies. Cell Mol Neurobiol. 2007; 27:943–957. PMID: 17965932.
Article24. Shibata N, Kobayashi M. The role for oxidative stress in neurodegenerative diseases. Brain Nerve. 2008; 60:157–170. PMID: 18306664.25. Aruoma OI, Bahorun T, Jen LS. Neuroprotection by bioactive components in medicinal and food plant extracts. Mutat Res. 2003; 544:203–215. PMID: 14644322.
Article26. Kitagishi Y, Nakanishi A, Ogura Y, Matsuda S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer's disease. Alzheimers Res Ther. 2014; 6:35. PMID: 25031641.
Article27. Zheng WX, Wang F, Cao XL, Pan HY, Liu XY, Hu XM, Sun YY. Baicalin protects PC-12 cells from oxidative stress induced by hydrogen peroxide via anti-apoptotic effects. Brain Inj. 2014; 28:227–234. PMID: 24456060.
Article28. Palacios-pelaez R, Lukiw WJ, Bazan NG. Omega-3 essential fatty acids modulate initiation and progression of neurodegenerative disease. Mol Neurobiol. 2010; 41:367–374. PMID: 20467837.
Article29. Chung KH, Hwang HJ, Shin KO, Jeon WM, Choi KS. Effects of perilla oil on plasma concentrations of cardioprotective (n-3) fatty acids and lipid profiles in mice. Nutr Res Pract. 2013; 7:256–261. PMID: 23964311.
Article30. Gerster H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res. 1998; 68:159–173. PMID: 9637947.31. Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N. Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. J Lipid Res. 2001; 42:1257–1265. PMID: 11483627.
Article32. Blondeau N, Tauskela JS. A new future in brain preconditioning based on nutraceuticals: a focus on α-linolenic omega-3 fatty acid for stroke protection. In : Gidday JM, Perez-Pinzon MA, Zhang JH, editors. Innate Tolerance in the CNS. New York (NY): Springer;2013. p. 133–163.33. Jang JY, Kim TS, Kim J, Kim Y, Shin K, Kim K, Lee SP, Kang MH, Choi EK, Ree MH, Kim YB. Perilla oil improves blood flow through inhibition of platelet aggregation and thrombus formation. Lab Anim Res. 2014; 30:21–27. PMID: 24707301.
Article34. Senavong P, Kongkham S, Saelim S, Suangkavathin V. Neuroprotective effect of perilla extracts on PC12 cells. J Med Assoc Thai. 2016; 99:S256–S264.
Article35. Lee AY, Lee MH, Lee S, Cho EJ. Comparative study on antioxidant activity of vegetable oils under in vitro and cellular system. J Agric Sci. 2015; 7:58–65.
Article36. Kim SS, Park RY, Jeon HJ, Kwon YS, Chun W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother Res. 2005; 19:243–245. PMID: 15934031.
Article37. Chetsawang J, Govitrapong P, Chetsawang B. Hydrogen peroxide toxicity induces Ras signaling in human neuroblastoma SH-SY5Y cultured cells. J Biomed Biotechnol. 2010; 2010:803815. PMID: 20871828.
Article38. Barbosa DS, Cecchini RE, Kadri MZ, Rodriguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil ω-3 fatty acids. Nutrition. 2003; 19:837–842. PMID: 14559317.
Article39. Fotakis G, Timbrell JA. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006; 160:171–177. PMID: 16111842.40. Wang P, Henning SM, Heber D. Limitation of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS One. 2010; 5:e10202. PMID: 20419137.41. Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998; 391:43–50. PMID: 9422506.
Article42. Kwon SH, Kim MJ, Ma SX, You IJ, Hwang JY, Oh JH, Kim SY, Kim HC, Lee SY, Jang CG. Eucommia ulmoides Oliv. Bark. protects against hydrogen peroxide-induced neuronal cell death in SH-SY5Y cells. J Ethnopharmacol. 2012; 142:337–345. PMID: 22735663.43. Hu XL, Niu YX, Zhang Q, Tian X, Gao LY, Guo LP, Meng WH, Zhao QC. Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ Toxicol Pharmacol. 2015; 40:230–240. PMID: 26164594.
Article44. Youle RJ, Strasser A. The Bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008; 9:47–59. PMID: 18097445.
Article45. Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997; 387:773–776. PMID: 9194558.
Article46. Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000; 1:120–130. PMID: 11253364.
Article47. Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000; 7:102–111. PMID: 10713725.
Article48. Grütter MG. Caspases: key players in programmed cell death. Curr Opin Struct Biol. 2000; 10:649–655. PMID: 11114501.
Article49. Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr. 2009; 139:861–868. PMID: 19261730.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Lipid Pneumonia by Green Perilla Oil
- Effects of perilla oil on plasma concentrations of cardioprotective (n-3) fatty acids and lipid profiles in mice
- Protective Role of Corticosterone against Hydrogen Peroxide-Induced Neuronal Cell Death in SH-SY5Y Cells
- Fatty Acid Content in Perilla Cultivars and Commercial Oils Determined by GC Analysis
- Neuroprotective Effects of Methanol Extracts of Jeju Native Plants on Hydrogen Peroxide-induced Cytotoxicity in SH-SY5Y Human Neuroblastoma Cells