Korean J Physiol Pharmacol.
2018 Mar;22(2):135-143. 10.4196/kjpp.2018.22.2.135.
Tumor necrosis factor α-converting enzyme inhibitor attenuates lipopolysaccharide-induced reactive oxygen species and mitogen-activated protein kinase expression in human renal proximal tubule epithelial cells
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju 61707, Korea. skimw@chonnam.ac.kr
- 2Global Desalination Research Center (GDRC), School of Environmental Science and Engineering (SESE), Gwangju Institute of Science and Technology (GIST), Gwnagju 61005, Korea.
- KMID: 2410103
- DOI: http://doi.org/10.4196/kjpp.2018.22.2.135
Abstract
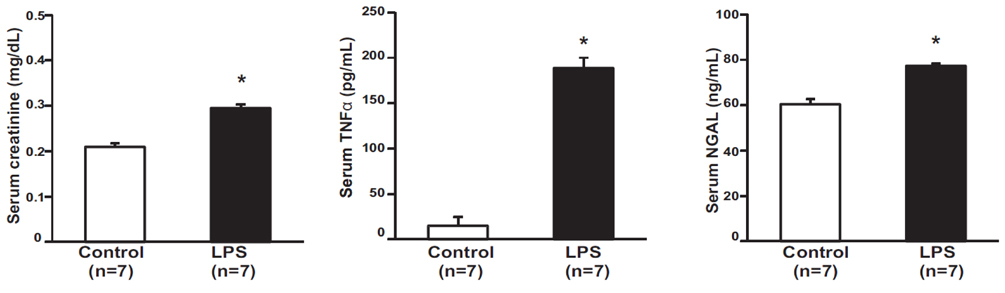
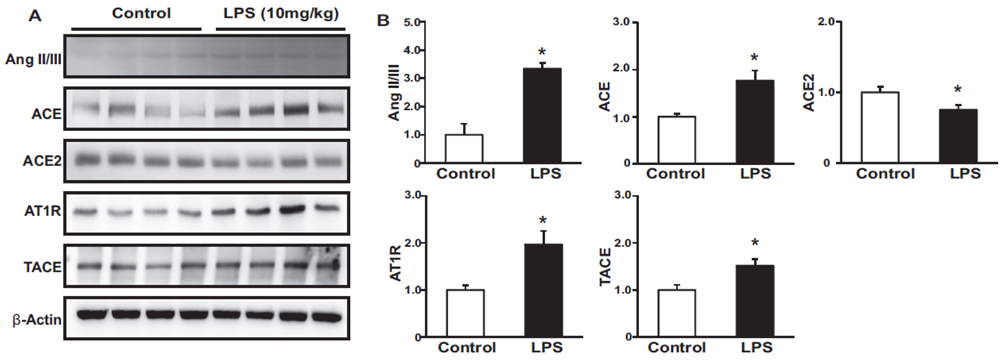
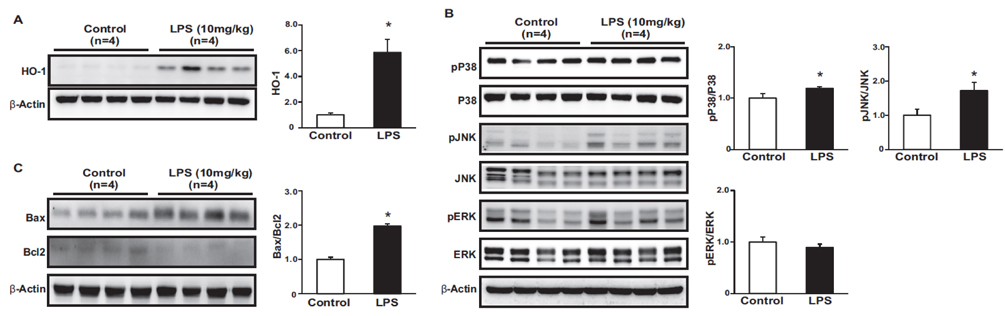
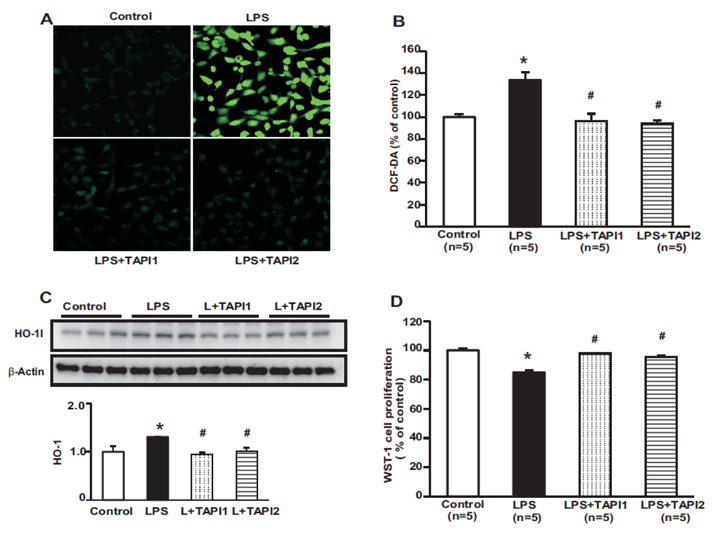
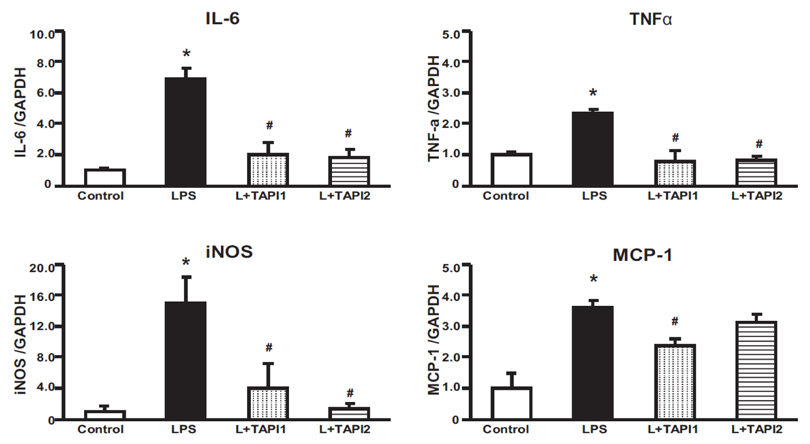
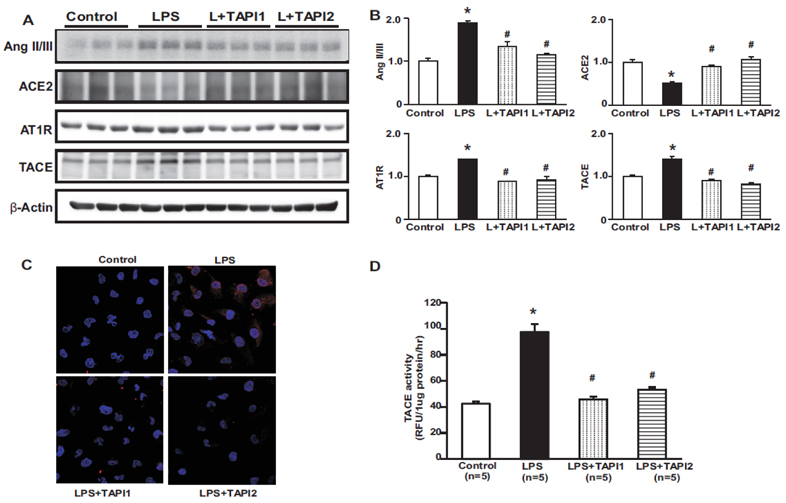
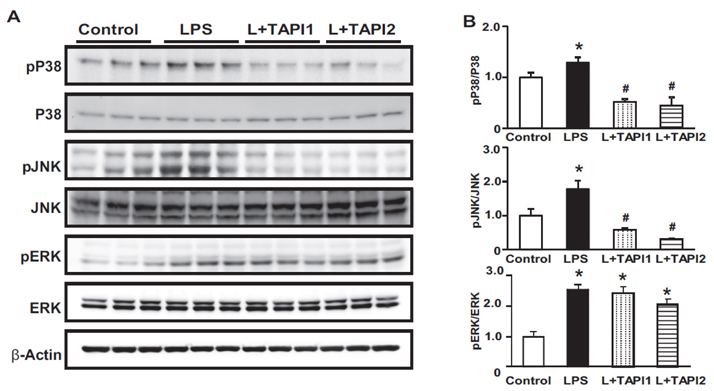
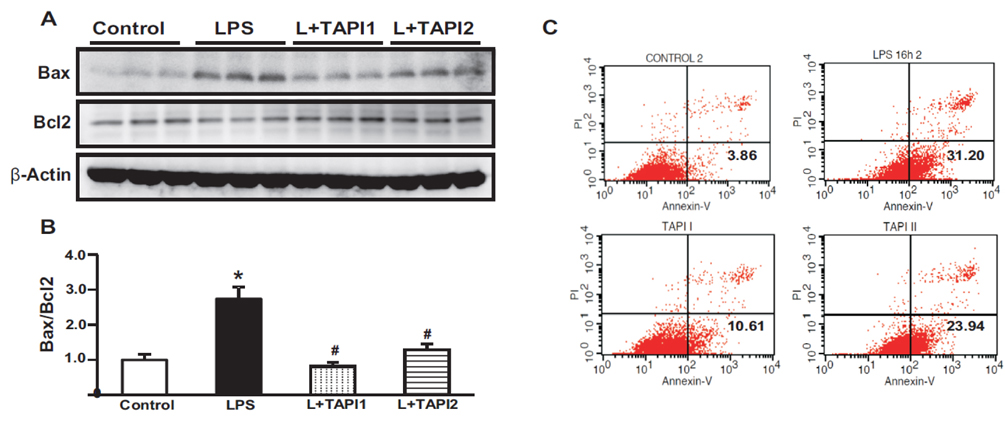
- Tumor necrosis factor-α (TNFα) and the angiotensin system are involved in inflammatory diseases and may contribute to acute kidney injury. We investigated the mechanisms by which TNFα-converting enzyme (TACE) contributes to lipopolysaccharide (LPS)-induced renal inflammation and the effect of TACE inhibitor treatment on LPS-induced cellular injury in human renal proximal tubule epithelial (HK-2) cells. Mice were treated with LPS (10 mg/kg, i.p.) and HK-2 cells were cultured with or without LPS (10 µg/ml) in the presence or absence of a type 1 TACE inhibitor (1 µM) or type 2 TACE inhibitor (10 µM). LPS treatment induced increased serum creatinine, TNFα, and urinary neutrophil gelatinase-associated lipocalin. Angiotensin II type 1 receptor, mitogen activated protein kinase (MAPK), and TACE increased, while angiotensin-converting enzyme-2 (ACE2) expression decreased in LPS-induced acute kidney injury and LPS-treated HK-2 cells. LPS induced reactive oxygen species and the down-regulation of ACE2, and these responses were prevented by TACE inhibitors in HK-2 cells. TACE inhibitors increased cell viability in LPS-treated HK-2 cells and attenuated oxidative stress and inflammatory cytokines. Our findings indicate that LPS activates renin angiotensin system components via the activation of TACE. Furthermore, inhibitors of TACE are potential therapeutic agents for kidney injury.
Keyword
MeSH Terms
-
Acute Kidney Injury
Angiotensins
Animals
Cell Survival
Creatinine
Cytokines
Down-Regulation
Epithelial Cells*
Humans*
Inflammation
Kidney
Lipocalins
Mice
Necrosis
Neutrophils
Oxidative Stress
Protein Kinases*
Reactive Oxygen Species*
Receptor, Angiotensin, Type 1
Renin-Angiotensin System
Tumor Necrosis Factor-alpha*
Angiotensins
Creatinine
Cytokines
Lipocalins
Protein Kinases
Reactive Oxygen Species
Receptor, Angiotensin, Type 1
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Kiemer AK, Müller C, Vollmar AM. Inhibition of LPS-induced nitric oxide and TNF-alpha production by alpha-lipoic acid in rat Kupffer cells and in RAW 264.7 murine macrophages. Immunol Cell Biol. 2002; 80:550–557.2. Goraca A, Józefowicz-Okonkwo G. Protective effects of early treatment with lipoic acid in LPS-induced lung injury in rats. J Physiol Pharmacol. 2007; 58:541–549.3. Goraca A, Piechota A, Huk-Kolega H. Effect of alpha-lipoic acid on LPS-induced oxidative stress in the heart. J Physiol Pharmacol. 2009; 60:61–68.4. Olesen ET, de Seigneux S, Wang G, Lütken SC, Frøkiaer J, Kwon TH, Nielsen S. Rapid and segmental specific dysregulation of AQP2, S256-pAQP2 and renal sodium transporters in rats with LPS-induced endotoxaemia. Nephrol Dial Transplant. 2009; 24:2338–2349.
Article5. Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993; 73:457–467.6. Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The role of ADAM-mediated shedding in vascular biology. Eur J Cell Biol. 2012; 91:472–485.
Article7. Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014; 66:167–176.
Article8. Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010; 59:529–538.
Article9. Liu Z, Huang XR, Chen HY, Penninger JM, Lan HY. Loss of angiotensin-converting enzyme 2 enhances TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab Invest. 2012; 92:650–661.
Article10. Fang F, Liu GC, Zhou X, Yang S, Reich HN, Williams V, Hu A, Pan J, Konvalinka A, Oudit GY, Scholey JW, John R. Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PLoS One. 2013; 8:e71433.
Article11. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, Kim SW. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am J Physiol Renal Physiol. 2010; 298:F301–F313.
Article12. Kassiri Z, Oudit GY, Sanchez O, Dawood F, Mohammed FF, Nuttall RK, Edwards DR, Liu PP, Backx PH, Khokha R. Combination of tumor necrosis factor-alpha ablation and matrix metalloproteinase inhibition prevents heart failure after pressure overload in tissue inhibitor of metalloproteinase-3 knock-out mice. Circ Res. 2005; 97:380–390.13. Kim CS, Joo SY, Lee KE, Choi JS, Bae EH, Ma SK, Kim SH, Lee J, Kim SW. Paricalcitol attenuates 4-hydroxy-2-hexenal-induced inflammation and epithelial-mesenchymal transition in human renal proximal tubular epithelial cells. PLoS One. 2013; 8:e63186.
Article14. Zamai L, Falcieri E, Marhefka G, Vitale M. Supravital exposure to propidium iodide identifies apoptotic cells in the absence of nucleosomal DNA fragmentation. Cytometry. 1996; 23:303–311.
Article15. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003; 3:745–756.
Article16. Asai M, Hattori C, Szabó B, Sasagawa N, Maruyama K, Tanuma S, Ishiura S. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem Biophys Res Commun. 2003; 301:231–235.17. Moss ML, Sklair-Tavron L, Nudelman R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008; 4:300–309.
Article18. Kataoka H. EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J Dermatol Sci. 2009; 56:148–153.
Article19. Bahia MS, Silakari O. Tumor necrosis factor alpha converting enzyme: an encouraging target for various inflammatory disorders. Chem Biol Drug Des. 2010; 75:415–443.
Article20. Hayashida K, Bartlett AH, Chen Y, Park PW. Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken). 2010; 293:925–937.
Article21. Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010; 45:146–169.
Article22. Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997; 385:729–733.23. Zhang Z, Kolls JK, Oliver P, Good D, Schwarzenberger PO, Joshi MS, Ponthier JL, Lancaster JR Jr. Activation of tumor necrosis factor-alpha-converting enzyme-mediated ectodomain shedding by nitric oxide. J Biol Chem. 2000; 275:15839–15844.24. Zhang Z, Oliver P, Lancaster JR Jr, Schwarzenberger PO, Joshi MS, Cork J, Kolls JK. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001; 15:303–305.
Article25. Pietri M, Schneider B, Mouillet-Richard S, Ermonval M, Mutel V, Launay JM, Kellermann O. Reactive oxygen species-dependent TNF-alpha converting enzyme activation through stimulation of 5-HT2B and alpha1D autoreceptors in neuronal cells. FASEB J. 2005; 19:1078–1087.26. Myers TJ, Brennaman LH, Stevenson M, Higashiyama S, Russell WE, Lee DC, Sunnarborg SW. Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-alpha shedding. Mol Biol Cell. 2009; 20:5236–5249.27. Blouin E, Halbwachs-Mecarelli L, Rieu P. Redox regulation of beta2-integrin CD11b/CD18 activation. Eur J Immunol. 1999; 29:3419–3431.28. Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005; 6:587–592.
Article29. Pedron T, Girard R, Chaby R. TLR4-dependent lipopolysaccharide-induced shedding of tumor necrosis factor receptors in mouse bone marrow granulocytes. J Biol Chem. 2003; 278:20555–20564.
Article30. Lovejoy B, Welch AR, Carr S, Luong C, Broka C, Hendricks RT, Campbell JA, Walker KA, Martin R, Van Wart H, Browner MF. Crystal structures of MMP-1 and -13 reveal the structural basis for selectivity of collagenase inhibitors. Nat Struct Biol. 1999; 6:217–221.31. Rosenau C, Emery D, Kaboord B, Qoronfleh MW. Development of a high-throughput plate-based chemiluminescent transcription factor assay. J Biomol Screen. 2004; 9:334–342.
Article32. Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol Rev. 2007; 220:8–21.
Article33. Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996; 384:368–372.
Article34. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516.
Article35. Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001; 31:1287–1312.36. Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000; 6:513–519.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyclosporin A aggravates hydrogen peroxide-induced cell death in kidney proximal tubule epithelial cells
- Mycobacterium tuberculosis Induces the Production of Tumor Necrosis Factor-alpha, Interleukin-6, and CXCL8 in Pulmonary Epithelial Cells Through Reactive Oxygen Species-dependent Mitogen-activated Protein Kinase Activation
- Role of Reactive Oxygen Species and Mitogen-activated Protein Kinases in 2, 3, 7, 8-tetrachlorodibinzo-p-dioxin-induced Fibronectin Secretion by MDCK Cells
- p38 Mitogen-Activated Protein Kinase and Extracellular Signal-Regulated Kinase Regulate Nitric Oxide Production and Inflammatory Cytokine Expression in Raw Cells
- Rottlerin enhances IL-1beta-induced COX-2 expression through sustained p38 MAPK activation in MDA-MB-231 human breast cancer cells