Anat Cell Biol.
2019 Sep;52(3):312-323. 10.5115/acb.18.192.
Cyclosporin A aggravates hydrogen peroxide-induced cell death in kidney proximal tubule epithelial cells
- Affiliations
-
- 1Interdisciplinary Graduate Program in Advanced Convergence Technology & Science, Jeju National University, Jeju, Korea. jinu.kim@jejunu.ac.kr
- 2Department of Anatomy, Jeju National University School of Medicine, Jeju, Korea.
- KMID: 2459547
- DOI: http://doi.org/10.5115/acb.18.192
Abstract
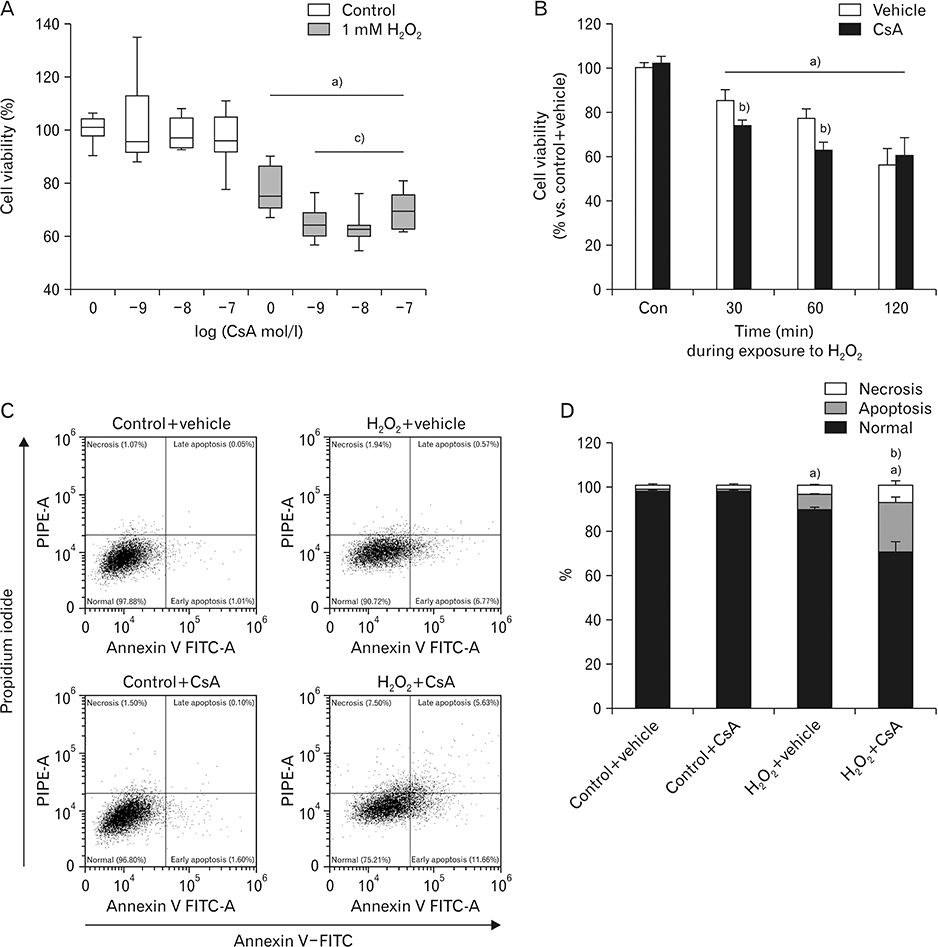
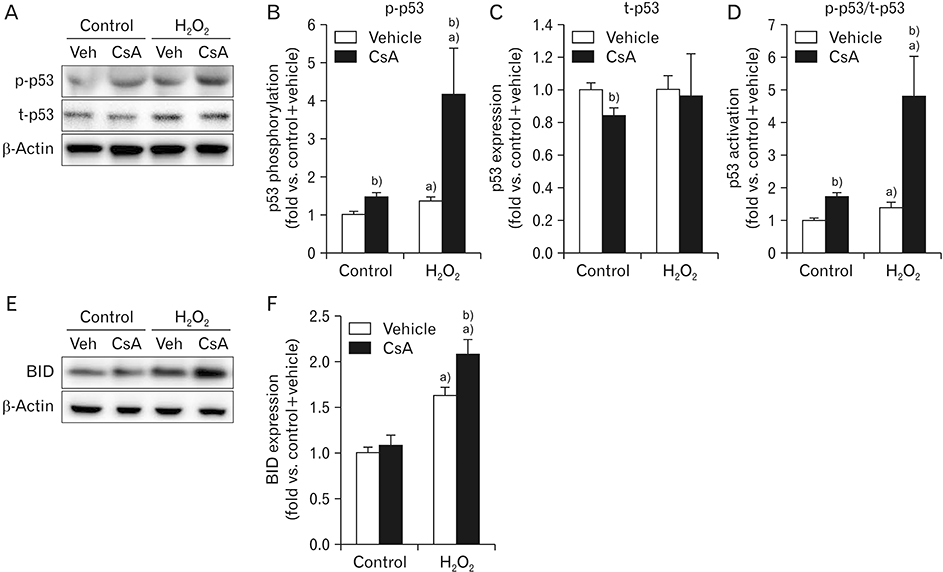
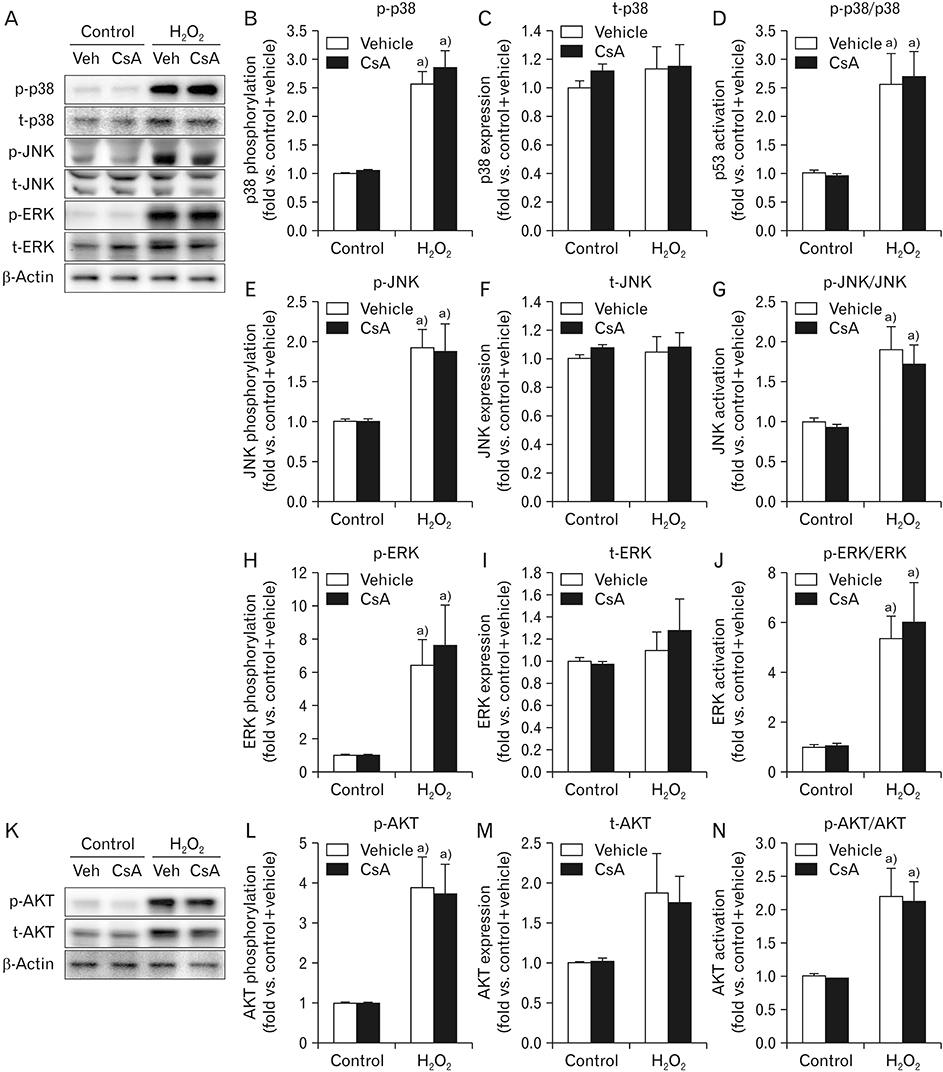
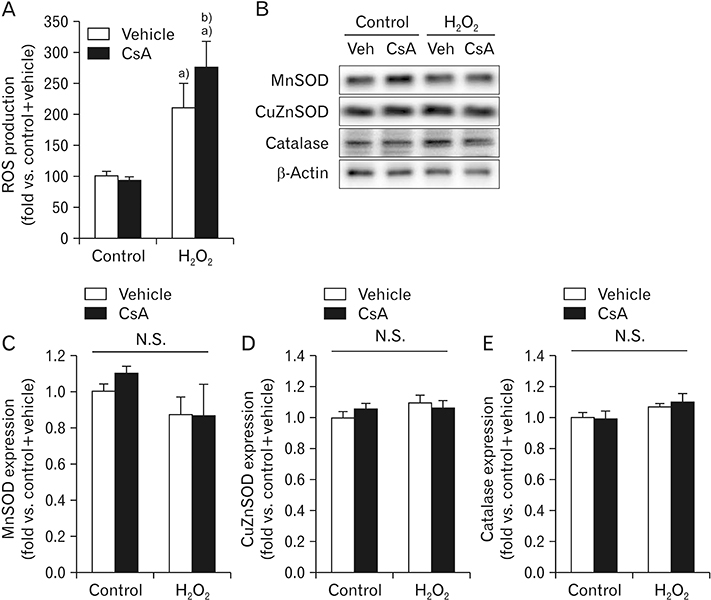
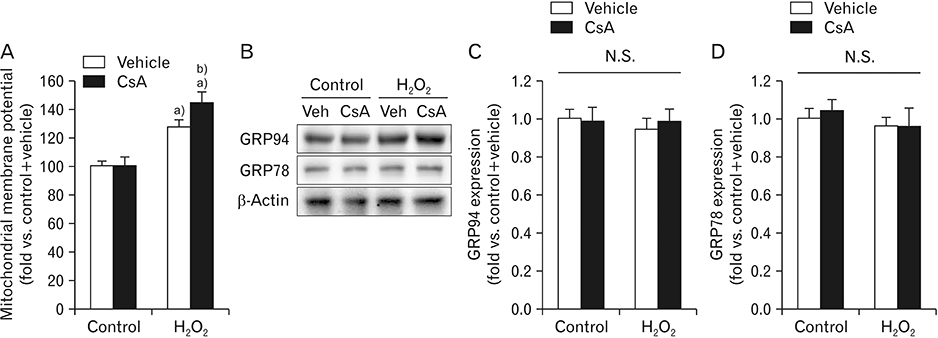
- Cyclosporin A (CsA) does not only exert a toxic effect on kidney parenchymal cells, but also protects them against necrotic cell death by inhibiting opening of mitochondrial permeability transition pore. However, whether CsA plays a role in hydrogen peroxide-induced kidney proximal tubular cell death is currently unclear. In the present study, treatment with CsA further increased apoptosis and necrosis in HK-2 human kidney proximal tubule epithelial cells during exposure to hydrogen peroxide. In addition, hydrogen peroxide-induced p53 activation and BH3 interacting-domain death agonist (BID) expression were higher in CsA-treated cells than those in non-treated cells, whereas hydrogen peroxide-induced activation of mitogen-activated protein kinases including p38, c-Jun N-terminal kinase, and extracellular signal-regulated kinase and activation of protein kinase B were not significantly altered by treatment with CsA. In oxidant-antioxidant system, reactive oxygen species (ROS) production induced by hydrogen peroxide was further enhanced by treatment with CsA. However, expression levels of antioxidant enzymes including manganese superoxide dismutase, copper/zinc superoxide dismutase, and catalase were not altered by treatment with hydrogen peroxide or CsA. Treatment with CsA further enhanced mitochondrial membrane potential induced by exposure to hydrogen peroxide, although it did not alter endoplasmic reticulum stress based on expression of glucose-regulated protein 78 and 94. Taken together, these data suggest that CsA can aggravate hydrogen peroxide-induced cell death through p53 activation, BID expression, and ROS production.
Keyword
MeSH Terms
-
Apoptosis
Catalase
Cell Death*
Cyclosporine*
Endoplasmic Reticulum Stress
Epithelial Cells*
Humans
Hydrogen Peroxide
Hydrogen*
JNK Mitogen-Activated Protein Kinases
Kidney*
Membrane Potential, Mitochondrial
Mitogen-Activated Protein Kinases
Necrosis
Permeability
Phosphotransferases
Proto-Oncogene Proteins c-akt
Reactive Oxygen Species
Superoxide Dismutase
Catalase
Cyclosporine
Hydrogen
Hydrogen Peroxide
JNK Mitogen-Activated Protein Kinases
Mitogen-Activated Protein Kinases
Phosphotransferases
Proto-Oncogene Proteins c-akt
Reactive Oxygen Species
Superoxide Dismutase
Figure
Reference
-
1. Borel JF, Feurer C, Gubler HU, Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976; 6:468–475.
Article2. White DJ, Calne RY. The use of cyclosporin A immunosuppression in organ grafting. Immunol Rev. 1982; 65:115–131.
Article3. Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989; 337:473–475.
Article4. Pereira MJ, Palming J, Rizell M, Aureliano M, Carvalho E, Svensson MK, Eriksson JW. The immunosuppressive agents rapamycin, cyclosporin A and tacrolimus increase lipolysis, inhibit lipid storage and alter expression of genes involved in lipid metabolism in human adipose tissue. Mol Cell Endocrinol. 2013; 365:260–269.
Article5. Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991; 23:1351–1354.
Article6. Bunjes D, Hardt C, Rollinghoff M, Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981; 11:657–661.
Article7. Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984; 311:699–705.
Article8. Thomas SE, Andoh TF, Pichler RH, Shankland SJ, Couser WG, Bennett WM, Johnson RJ. Accelerated apoptosis characterizes cyclosporine-associated interstitial fibrosis. Kidney Int. 1998; 53:897–908.
Article9. Young BA, Burdmann EA, Johnson RJ, Alpers CE, Giachelli CM, Eng E, Andoh T, Bennett WM, Couser WG. Cellular proliferation and macrophage influx precede interstitial fibrosis in cyclosporine nephrotoxicity. Kidney Int. 1995; 48:439–448.
Article10. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005; 434:658–662.
Article11. Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010; 48:749–762.
Article12. Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxideinduced renal injury: a protective role for pyruvate in vitro and in vivo. J Clin Invest. 1991; 88:1886–1893.13. Kim J, Kil IS, Seok YM, Yang ES, Kim DK, Lim DG, Park JW, Bonventre JV, Park KM. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice: a role for manganese superoxide dismutase. J Biol Chem. 2006; 281:20349–20356.14. Kim J, Kim KY, Jang HS, Yoshida T, Tsuchiya K, Nitta K, Park JW, Bonventre JV, Park KM. Role of cytosolic NADP+-dependent isocitrate dehydrogenase in ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2009; 296:F622–F633.
Article15. Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009; 297:F461–F470.
Article16. Kim J, Long KE, Tang K, Padanilam BJ. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012; 82:193–203.
Article17. Park S, Yoon SP, Kim J. Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells. Anat Cell Biol. 2015; 48:66–74.
Article18. Yoon SP, Kim J. Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity. Anat Cell Biol. 2018; 51:189–199.
Article19. Kim J, Imig JD, Yang J, Hammock BD, Padanilam BJ. Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am J Physiol Renal Physiol. 2014; 307:F971–F980.
Article20. Kim J, Padanilam BJ. Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2011; 301:F450–F459.
Article21. Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013; 24:229–242.
Article22. Kim J, Yoon SP, Toews ML, Imig JD, Hwang SH, Hammock BD, Padanilam BJ. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015; 308:F131–F139.
Article23. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008; 73:994–1007.
Article24. Yoon SP, Kim J. Exogenous CGRP upregulates profibrogenic growth factors through PKC/JNK signaling pathway in kidney proximal tubular cells. Cell Biol Toxicol. 2018; 34:251–262.
Article25. Kim J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch Pharm Res. 2017; 40:1197–1208.
Article26. Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015; 87:350–358.
Article27. Ammar NM, El-Hawary SS, Mohamed DA, Afifi MS, Ghanem DM, Awad G. Phytochemical and biological studies of Tribulus terrestris L. growing in Egypt. Int J Pharmacol. 2018; 14:248–259.28. Ali A, Kim MJ, Kim MY, Lee HJ, Roh GS, Kim HJ, Cho GJ, Choi WS. Quercetin induces cell death in cervical cancer by reducing O-GlcNAcylation of adenosine monophosphate-activated protein kinase. Anat Cell Biol. 2018; 51:274–283.
Article29. Kim J. Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation. Anat Cell Biol. 2017; 50:200–206.
Article30. Kim J. Poly(ADP-ribose) polymerase activation induces high mobility group box 1 release from proximal tubular cells during cisplatin nephrotoxicity. Physiol Res. 2016; 65:333–340.
Article31. Lee JS, Lim JY, Kim J. Mechanical stretch induces angiotensinogen expression through PARP1 activation in kidney proximal tubular cells. In Vitro Cell Dev Biol Anim. 2015; 51:72–78.
Article32. Park Y, Tae HJ, Cho JH, Kim IS, Ohk TG, Park CW, Moon JB, Shin MC, Lee TK, Lee JC, Park JH, Ahn JH, Kang SH, Won MH, Cho JH. The relationship between low survival and acute increase of tumor necrosis factor alpha expression in the lung in a rat model of asphyxial cardiac arrest. Anat Cell Biol. 2018; 51:128–135.33. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–616.34. Song H, Yoon SP, Kim J. Poly(ADP-ribose) polymerase regulates glycolytic activity in kidney proximal tubule epithelial cells. Anat Cell Biol. 2016; 49:79–87.
Article35. Yoon SP, Kim J. Poly(ADP-ribose) polymerase 1 contributes to oxidative stress through downregulation of sirtuin 3 during cisplatin nephrotoxicity. Anat Cell Biol. 2016; 49:165–176.
Article36. Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L. L-carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept. 2010; 161:58–66.
Article37. Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012; 149:1536–1548.
Article38. Pyrzynska B, Serrano M, Martínez-A C, Kaminska B. Tumor suppressor p53 mediates apoptotic cell death triggered by cyclosporin A. J Biol Chem. 2002; 277:14102–14108.
Article39. Sugie N, Fujii N, Danno K. Cyclosporin-A suppresses p53-dependent repair DNA synthesis and apoptosis following ultraviolet-B irradiation. Photodermatol Photoimmunol Photomed. 2002; 18:163–168.
Article40. Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isono M, Isshiki K, Uzu T, Kashiwagi A, Koya D. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic Biol Med. 2006; 40:2175–2182.
Article41. Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW, Vogelstein B. Identification and classification of p53-regulated genes. Proc Natl Acad Sci U S A. 1999; 96:14517–14522.
Article42. Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996; 271:4138–4142.43. Han W, Ming M, He TC, He YY. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem. 2010; 285:11369–11377.44. Andreucci M, Fuiano G, Presta P, Lucisano G, Leone F, Fuiano L, Bisesti V, Esposito P, Russo D, Memoli B, Faga T, Michael A. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009; 42:554–561.
Article45. Wolf A, Clemann N, Frieauff W, Ryffel B, Cordier A. Role of reactive oxygen formation in the cyclosporin-A-mediated impairment of renal functions. Transplant Proc. 1994; 26:2902–2907.46. Hokanson JF, Mercier JG, Brooks GA. Cyclosporine A decreases rat skeletal muscle mitochondrial respiration in vitro. Am J Respir Crit Care Med. 1995; 151:1848–1851.47. Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007; 42:150–158.
Article48. Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, Ramachandrarao SP, Sharma K, Kurosaki T, Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005; 170:1079–1090.
Article49. Yoon SP, Kim J. Poly(ADP-ribose) polymerase 1 activation links ischemic acute kidney injury to interstitial fibrosis. J Physiol Sci. 2015; 65:105–111.
Article50. Kim J, Kim JI, Na YK, Park KM. Intra-renal slow cell-cycle cells contribute to the restoration of kidney tubules injured by ischemia/reperfusion. Anat Cell Biol. 2011; 44:186–193.
Article51. Kim J, Kim JI, Jang HS, Park JW, Park KM. Protective role of cytosolic NADP(+)-dependent isocitrate dehydrogenase, IDH1, in ischemic pre-conditioned kidney in mice. Free Radic Res. 2011; 45:759–766.
Article52. Niwa K, Inanami O, Yamamori T, Ohta T, Hamasu T, Kuwabara M. Redox regulation of PI3K/Akt and p53 in bovine aortic endothelial cells exposed to hydrogen peroxide. Antioxid Redox Signal. 2003; 5:713–722.
Article53. Wu GS. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol Ther. 2004; 3:156–161.54. Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem. 2001; 276:23357–23361.
Article55. Yoshioka T, Bills T, Moore-Jarrett T, Greene HL, Burr IM, Ichikawa I. Role of intrinsic antioxidant enzymes in renal oxidant injury. Kidney Int. 1990; 38:282–288.
Article56. Takeyama N, Miki S, Hirakawa A, Tanaka T. Role of the mitochondrial permeability transition and cytochrome C release in hydrogen peroxide-induced apoptosis. Exp Cell Res. 2002; 274:16–24.
Article57. Chen QM, Liu J, Merrett JB. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000; 347(Pt 2):543–551.
Article58. Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997; 88:323–331.
Article59. Voskamp P, Bodmann CA, Koehl GE, Rebel HG, Van Olderen MG, Gaumann A, El Ghalbzouri A, Tensen CP, Bavinck JN, Willemze R, Geissler EK, De Gruijl FR. Dietary immunosuppressants do not enhance UV-induced skin carcinogenesis, and reveal discordance between p53-mutant early clones and carcinomas. Cancer Prev Res (Phila). 2013; 6:129–138.
Article60. Yin Y, Terauchi Y, Solomon GG, Aizawa S, Rangarajan PN, Yazaki Y, Kadowaki T, Barrett JC. Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Nature. 1998; 391:707–710.
Article61. Mohamadin AM, El-Beshbishy HA, El-Mahdy MA. Green tea extract attenuates cyclosporine A-induced oxidative stress in rats. Pharmacol Res. 2005; 51:51–57.
Article62. Satyanarayana PS, Singh D, Chopra K. Quercetin, a bioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in rats. Methods Find Exp Clin Pharmacol. 2001; 23:175–181.
Article63. Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997; 90:595–606.
Article64. Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005; 280:18558–18561.
Article65. Wang C, Salahudeen AK. Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int. 1995; 47:927–934.
Article66. Longoni B, Migliori M, Ferretti A, Origlia N, Panichi V, Boggi U, Filippi C, Cuttano MG, Giovannini L, Mosca F. Melatonin prevents cyclosporine-induced nephrotoxicity in isolated and perfused rat kidney. Free Radic Res. 2002; 36:357–363.
Article67. Wang C, Salahudeen AK. Cyclosporine nephrotoxicity: attenuation by an antioxidant-inhibitor of lipid peroxidation in vitro and in vivo. Transplantation. 1994; 58:940–946.68. Longoni B, Boschi E, Demontis GC, Marchiafava PL, Mosca F. Regulation of Bcl-2 protein expression during oxidative stress in neuronal and in endothelial cells. Biochem Biophys Res Commun. 1999; 260:522–526.
Article69. Kim J, Jung KJ, Park KM. Reactive oxygen species differently regulate renal tubular epithelial and interstitial cell proliferation after ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2010; 298:F1118–F1129.
Article70. Kim J, Jang HS, Park KM. Reactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in mice. Am J Physiol Renal Physiol. 2010; 298:F158–F166.
Article71. Kim J, Park JW, Park KM. Increased superoxide formation induced by irradiation preconditioning triggers kidney resistance to ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2009; 296:F1202–F1211.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extraneural CGRP Induces Oxidative Stress in Kidney Proximal Tubule Epithelial Cells
- Protective effects of propofol against hydrogen peroxide-induced oxidative stress in human kidney proximal tubular cells
- Effects of Glutathione on Reactive Oxygen Species-Induced Cytotoxicity in Human Retinal Pigment Epithelial Cell Line
- Hydrogen Peroxide Upregulates TNF-Related Apoptosis-Inducing Ligand (TRAIL) Expression in Human Astroglial Cells, and Augments Apoptosis of T Cells
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells