Cancer Res Treat.
2017 Jan;49(1):230-245. 10.4143/crt.2015.506.
Studying the Effect of Downregulating Autophagy-Related Gene LC3 on TLR3 Apoptotic Pathway Mediated by dsRNA in Hepatocellular Carcinoma Cells
- Affiliations
-
- 1Department of Pathological Anatomy, Nantong University, Nantong, China. bl1@ntu.edu.cn
- KMID: 2367521
- DOI: http://doi.org/10.4143/crt.2015.506
Abstract
- PURPOSE
The purpose of this study is to examine the role of the double-stranded RNA (dsRNA) activated Toll-interleukin-1 receptor domain-containing adaptor inducing interferon β (TRIF) signal pathway in triggering apoptosis in hepatocellular carcinoma (HCC) cells.
MATERIALS AND METHODS
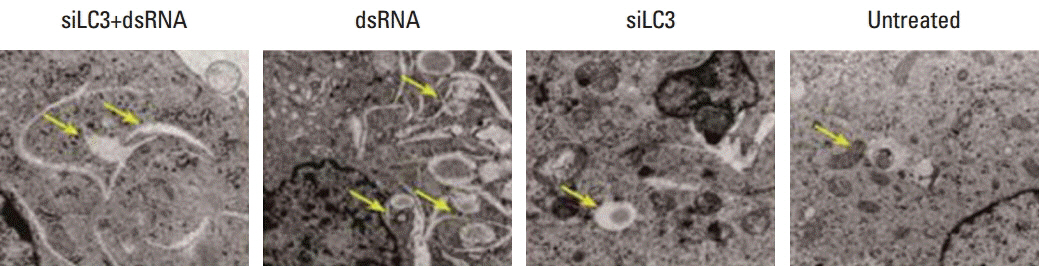
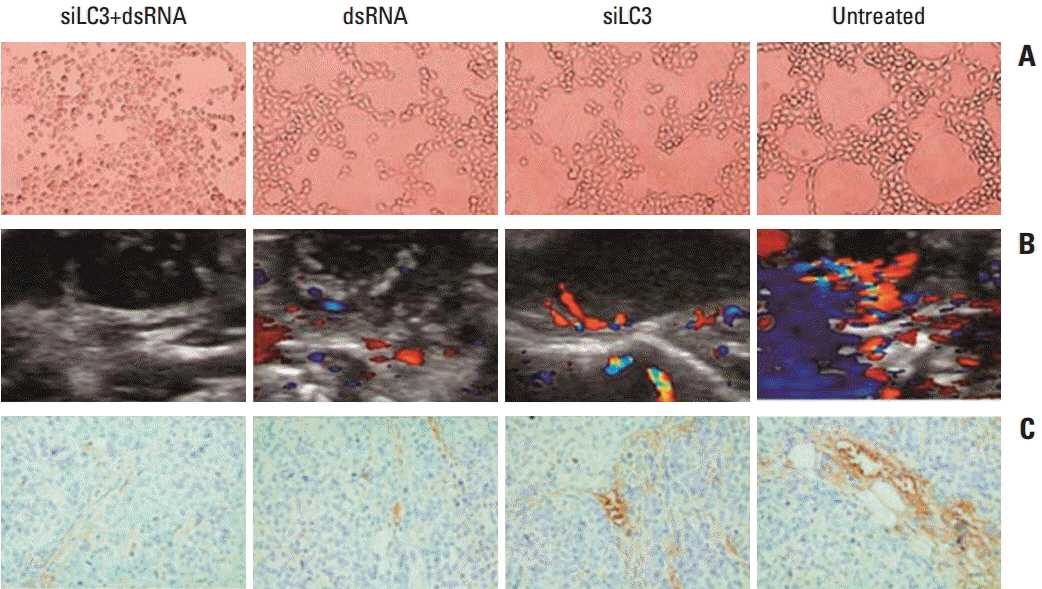
First, siRNA targeted autophagy-related gene LC3 (pU6H1-LC3 siRNA and siLC3) and a dsRNA used as a Toll-like receptor 3 (TLR3) ligand was constructed and synthesized, respectively. Then, a human HCC cell line was transfected with dsRNA, siLC3, and cotransfected with siLC3 and dsRNA (siLC3+dsRNA), respectively. Finally, quantification real-time polymerase chain reaction, western blotting, and immunofluorescence staining were used in the HCC line (SMMC7721), and MTT assay, flow cytometry, terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling, and transmission electron microscopy were used in an HCC xenograft model of nude mice. Human umbilical vein endothelial cell tube forming assay, color Doppler ultrasonographic flow image examination, and CD34-positive microvessel density were used in vitro and in vivo.
RESULTS
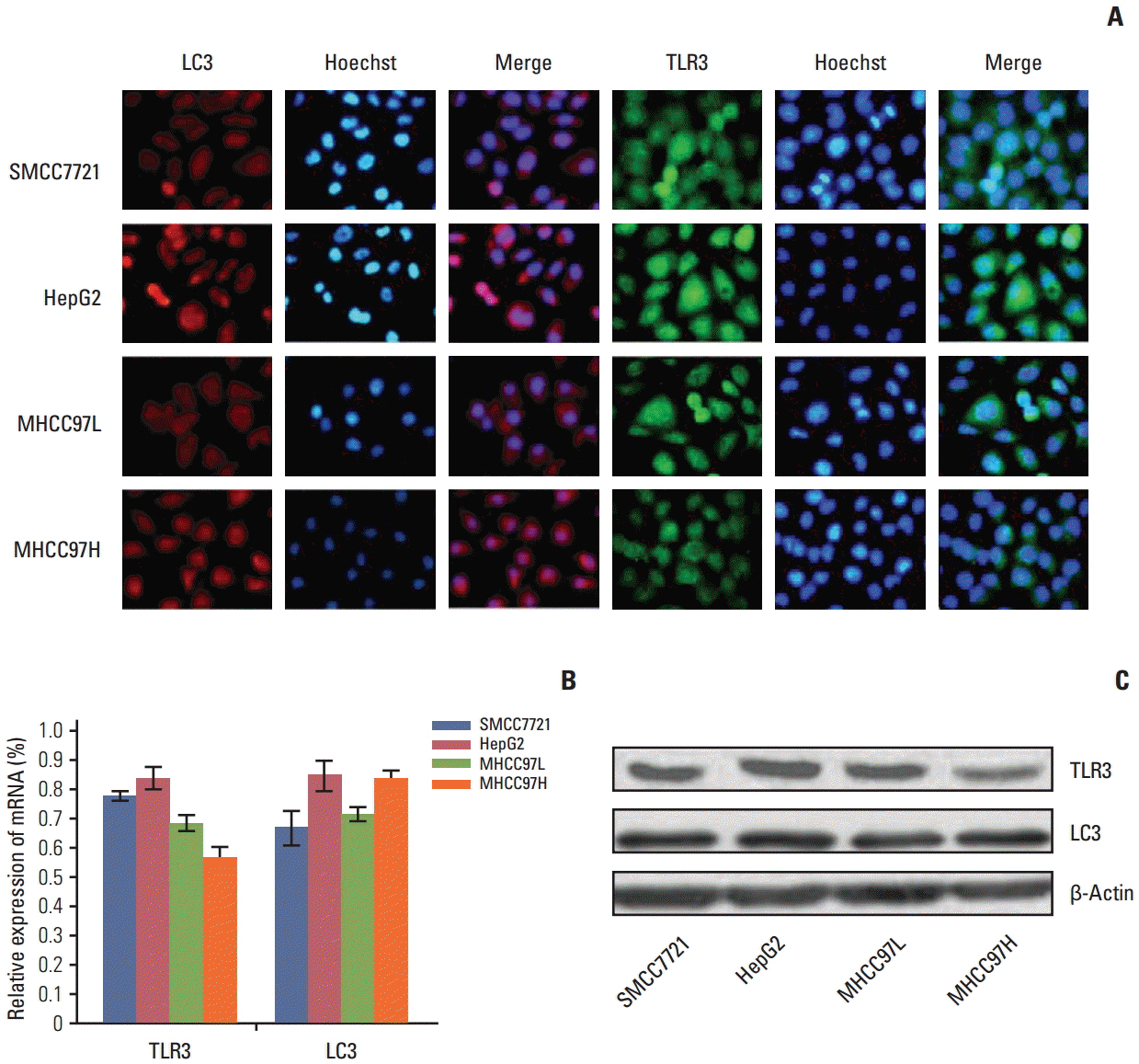
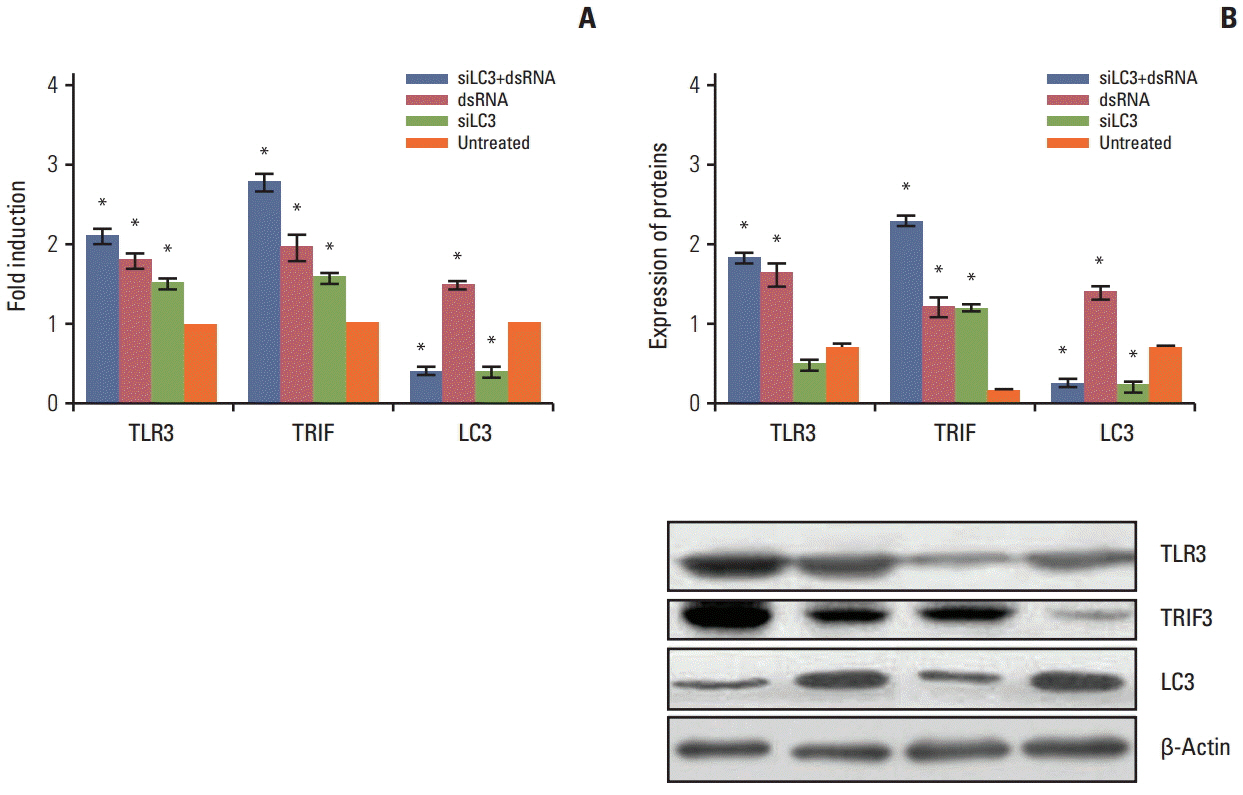
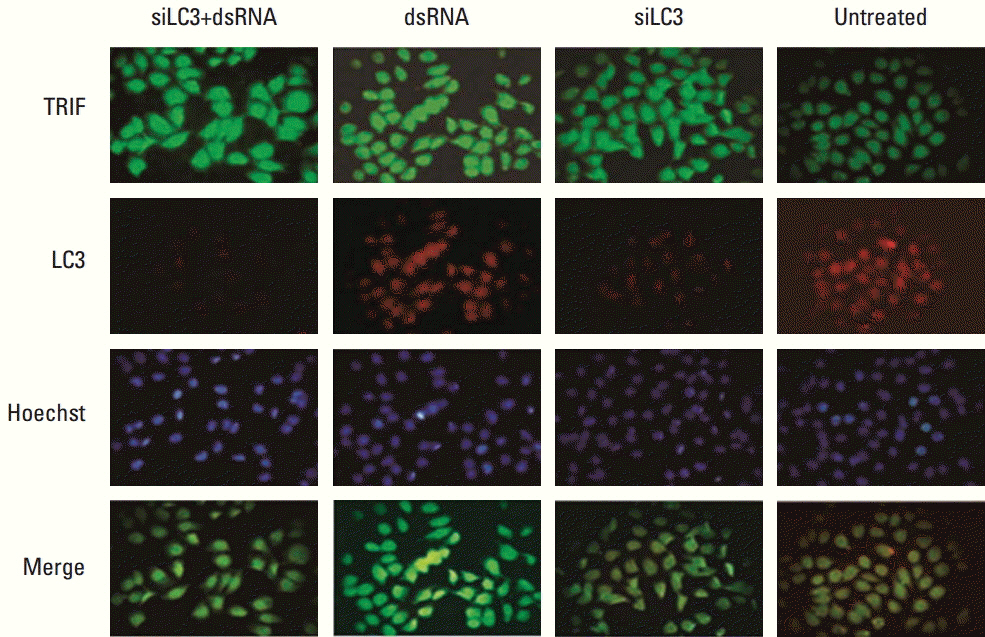
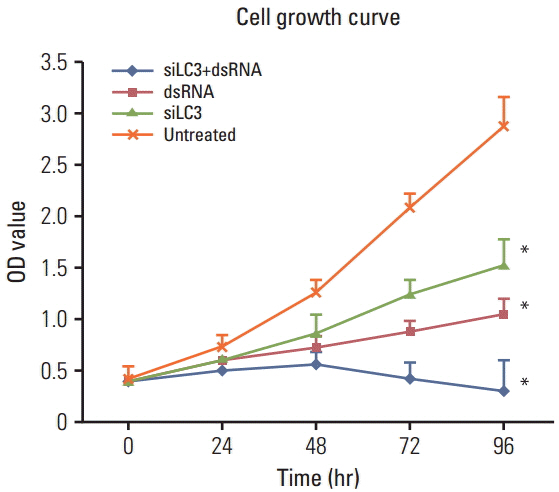
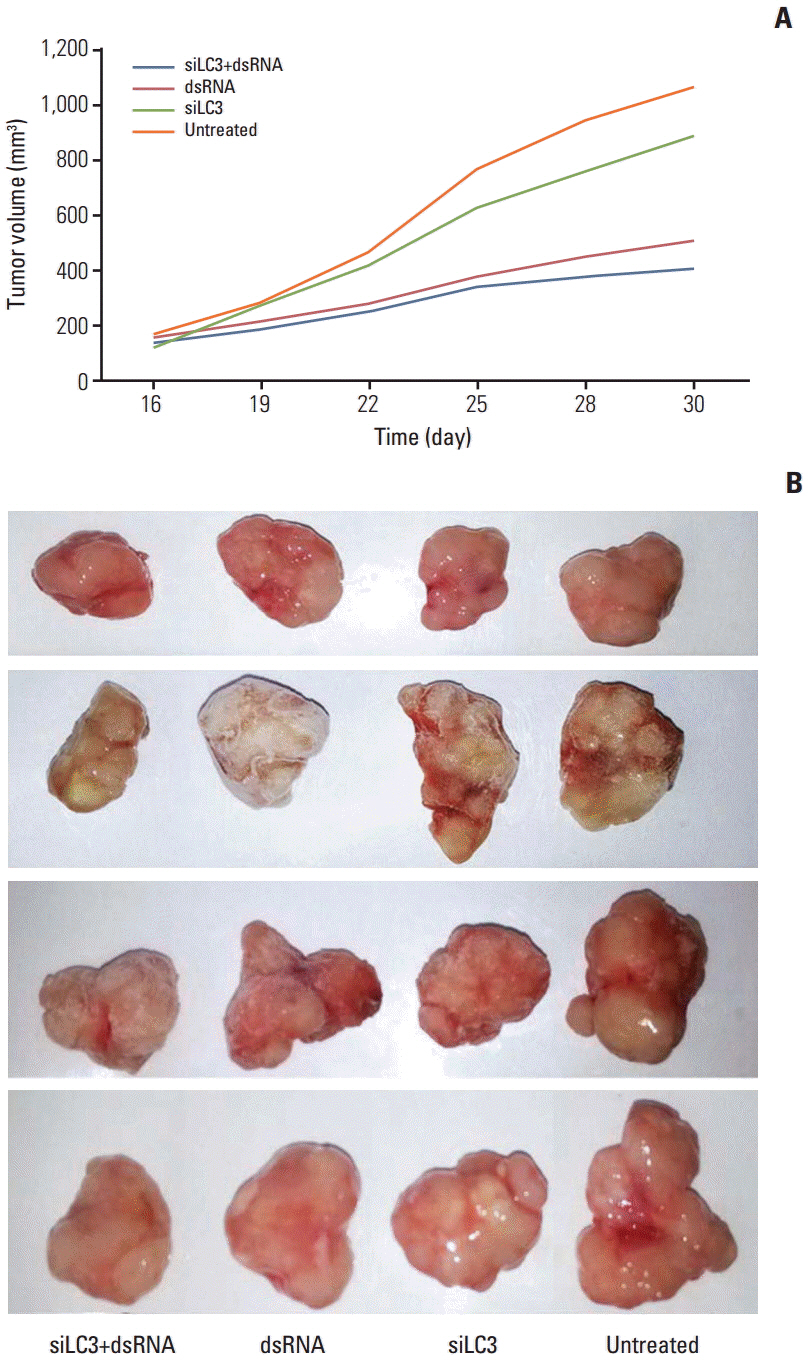
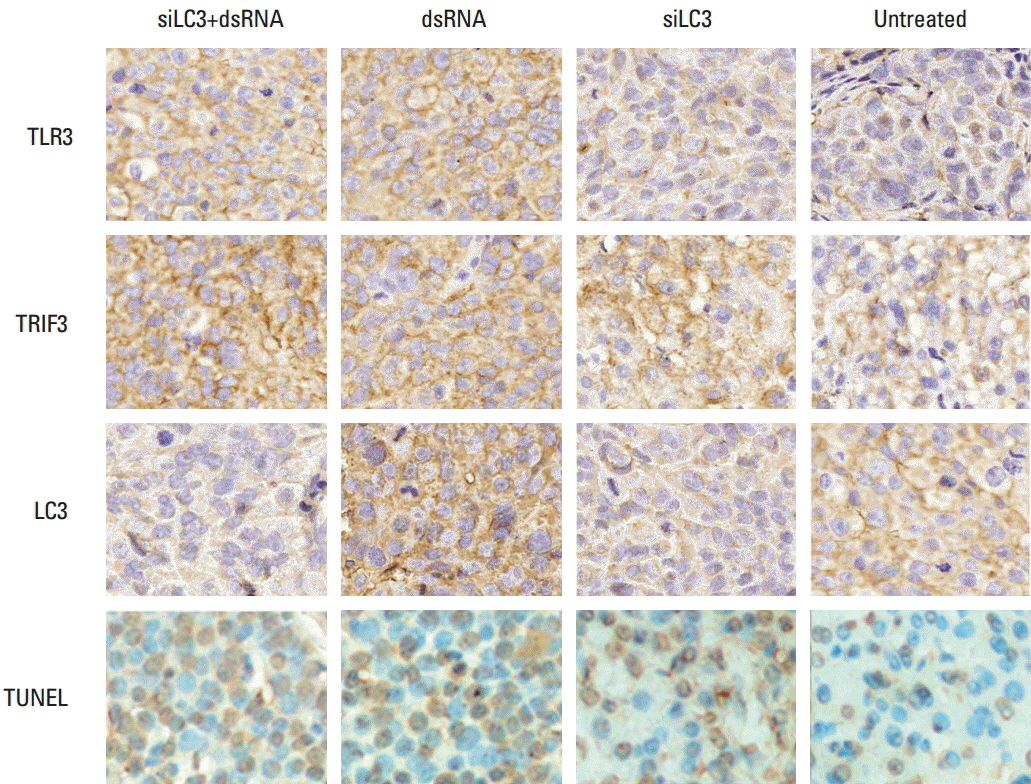
Compared with untreated cells, the protein and mRNA expression of TLR3 and TRIF was up-regulated, in order, siLC3+dsRNA, dsRNA, and siLC3. Expression of LC3 was obviously down-regulated and the autophagosomes were significantly decreased in siLC3+dsRNA and siLC3, whereas in dsRNA (p < 0.05). LC3 and TRIF colocation was observed in HepG2 cells. Decreased cell viability, increased apoptosis, decrease in xenograft tumor volume, and angiogenesis potential were also observed in order (p < 0.05).
CONCLUSION
Suppression of intracellular autophagy resulted in decreased degradation of TRIF protein, which can promote triggering of apoptosis by the TLR3-TRIF pathway. dsRNA and siLC3 could play anticancer roles in coordination.
Keyword
MeSH Terms
-
Animals
Apoptosis
Autophagy
Blotting, Western
Carcinoma, Hepatocellular*
Cell Line
Cell Survival
Endothelial Cells
Flow Cytometry
Fluorescent Antibody Technique
Hep G2 Cells
Heterografts
Humans
In Vitro Techniques
Interferons
Mice
Mice, Nude
Microscopy, Electron, Transmission
Microvessels
Real-Time Polymerase Chain Reaction
RNA, Double-Stranded
RNA, Messenger
RNA, Small Interfering
Signal Transduction
Toll-Like Receptor 3
Tumor Burden
Umbilical Veins
Interferons
RNA, Double-Stranded
RNA, Messenger
RNA, Small Interfering
Toll-Like Receptor 3
Figure
Reference
-
References
1. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000; 19:5720–8.
Article2. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011; 469:323–35.
Article3. Chen GY, Yang HJ, Lu CH, Chao YC, Hwang SM, Chen CL, et al. Simultaneous induction of autophagy and toll-like receptor signaling pathways by graphene oxide. Biomaterials. 2012; 33:6559–69.
Article4. Chen L, Xu YY, Zhou JM, Wu YY, E Q, Zhu YY. TLR3 dsRNA agonist inhibits growth and invasion of HepG2.2.15 HCC cells. Oncol Rep. 2012; 28:200–6.
Article5. Han KJ, Su X, Xu LG, Bin LH, Zhang J, Shu HB. Mechanisms of the TRIF-induced interferon-stimulated response element and NF-kappaB activation and apoptosis pathways. J Biol Chem. 2004; 279:15652–61.6. McAllister CS, Lakhdari O, Pineton de Chambrun G, Gareau MG, Broquet A, Lee GH, et al. TLR3, TRIF, and caspase 8 determine double-stranded RNA-induced epithelial cell death and survival in vivo. J Immunol. 2013; 190:418–27.
Article7. Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011; 17:654–66.
Article8. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010; 140:313–26.
Article9. Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008; 9:1004–10.
Article10. Inomata M, Niida S, Shibata K, Into T. Regulation of Toll-like receptor signaling by NDP52-mediated selective autophagy is normally inactivated by A20. Cell Mol Life Sci. 2012; 69:963–79.
Article11. Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007; 27:135–44.
Article12. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–84.
Article13. Mizushima N. Autophagy: process and function. Genes Dev. 2007; 21:2861–73.
Article14. Biswas N, Liu S, Ronni T, Aussenberg SE, Liu W, Fujita T, et al. The ubiquitin-like protein PLIC-1 or ubiquilin 1 inhibits TLR3-Trif signaling. PLoS One. 2011; 6:e21153.
Article15. Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006; 176:4894–901.
Article16. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140:805–20.
Article17. Yamamoto M, Takeda K. Current views of toll-like receptor signaling pathways. Gastroenterol Res Pract. 2010; 2010:240365.
Article18. Yang Y, Liao B, Wang S, Yan B, Jin Y, Shu HB, et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc Natl Acad Sci U S A. 2013; 110:5115–20.
Article19. Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011; 10:1533–41.
Article20. Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008; 27:1110–21.
Article21. Guo Z, Chen L, Zhu Y, Zhang Y, He S, Qin J, et al. Double-stranded RNA-induced TLR3 activation inhibits angiogenesis and triggers apoptosis of human hepatocellular carcinoma cells. Oncol Rep. 2012; 27:396–402.
Article22. Xu YY, Chen L, Zhou JM, Wu YY, Zhu YY. Inhibitory effect of dsRNA TLR3 agonist in a rat hepatocellular carcinoma model. Mol Med Rep. 2013; 8:1037–42.
Article23. Shi CS, Kehrl JH. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem. 2008; 283:33175–82.
Article24. Nomi N, Kodama S, Suzuki M. Toll-like receptor 3 signaling induces apoptosis in human head and neck cancer via survivin associated pathway. Oncol Rep. 2010; 24:225–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway
- Transglutaminase 2 Promotes Autophagy by LC3 Induction through p53 Depletion in Cancer Cell
- Clusterin Protects Lipotoxicity-Induced Apoptosis via Upregulation of Autophagy in Insulin-Secreting Cells
- High Glucose Accelerates Autophagy in Adult Rat Intervertebral Disc Cells
- Glutamine synthetase mediates sorafenib sensitivity in β-catenin-active hepatocellular carcinoma cells