Allergy Asthma Immunol Res.
2015 May;7(3):256-264. 10.4168/aair.2015.7.3.256.
Antiallergic Function of KR62980, a Peroxisome Proliferator-Activated Receptor-gamma Agonist, in a Mouse Allergic Rhinitis Model
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Chosun University College of Medicine, Gwangju, Korea.
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Hospital, Seoul, Korea.
- 3Sensory Organ Research Center, Seoul National University Biomedical Research Institute, Seoul, Korea. csrhee@snu.ac.kr
- 4Institute of Allergy and Clinical Immunology, Seoul National University Biomedical Research Institute, Seoul, Korea.
- 5Graduate School of Immunology, Seoul National University, Seoul, Korea.
- 6Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- KMID: 2260463
- DOI: http://doi.org/10.4168/aair.2015.7.3.256
Abstract
- PURPOSE
Peroxisome proliferator-activated receptor gamma (PPAR-gamma) has been shown to play an important role in the control of inflammatory responses acting on macrophages, mast cells, T cells and eosinophils. A novel PPAR-gamma ligand, KR62980 have been recently focused on due to the lower undesirable effects than other PPAR-gamma ligands such as rosiglitazone and pioglitazone. The present study was aimed to investigate the effects of KR62980 on nasal symptoms and immunopathological profiles in allergic nasal mucosa in murine allergic rhinitis model.
METHODS
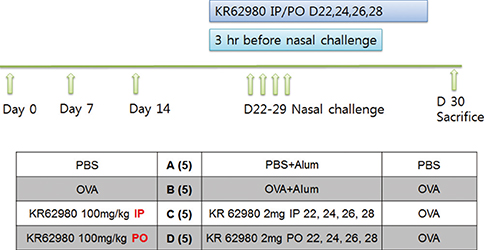
BALB/c mice were sensitized and challenged intranasally with ovalbumin (OVA). KR62980 was administered intraperitoneally or orally 3 hours before each intranasal OVA challenge.
RESULTS
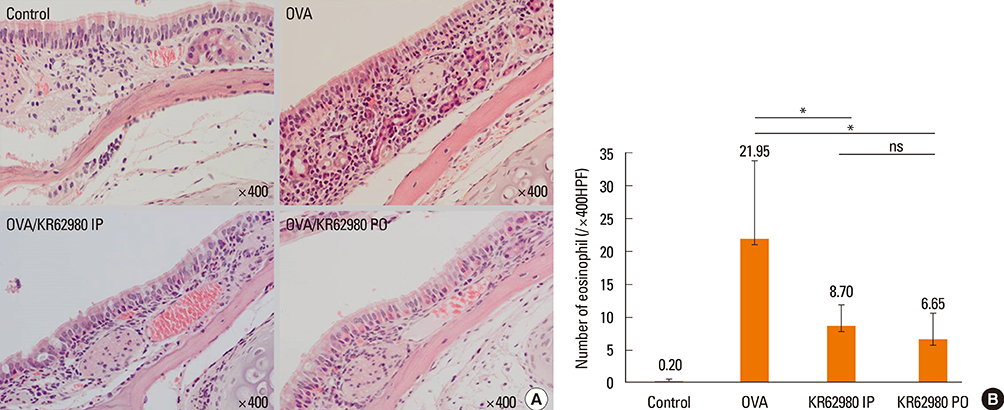
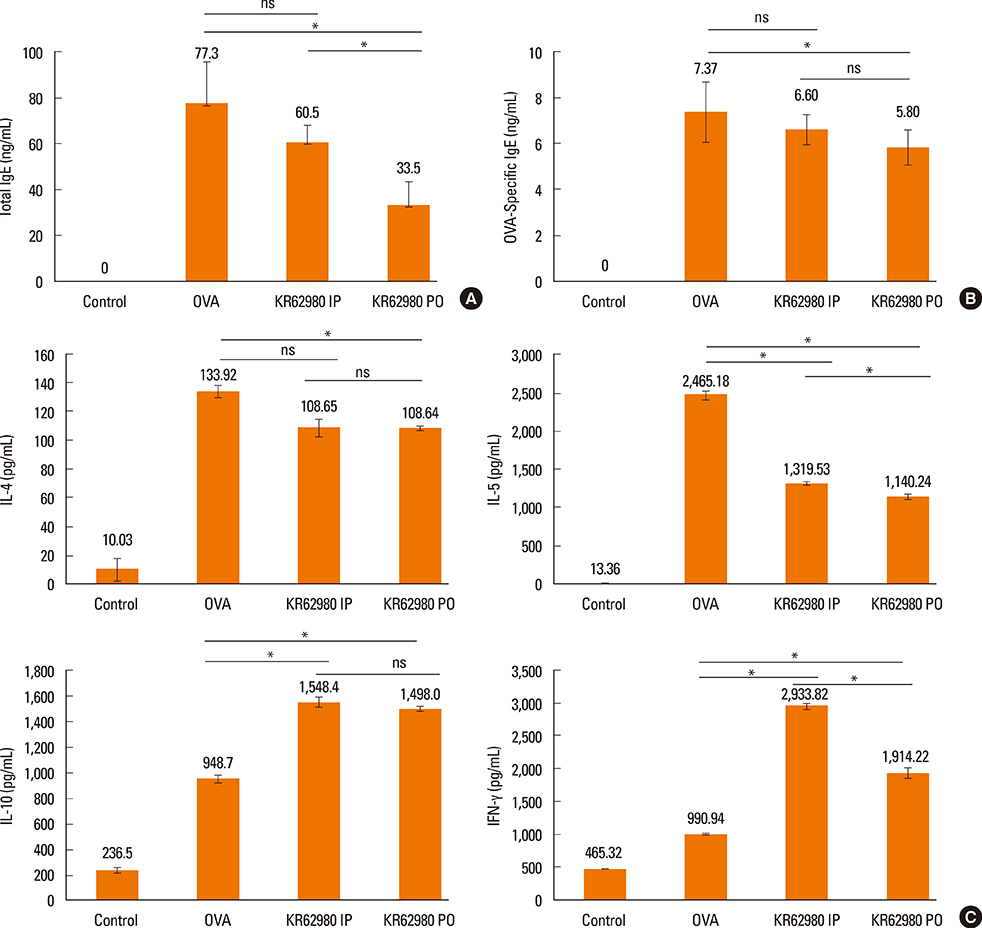
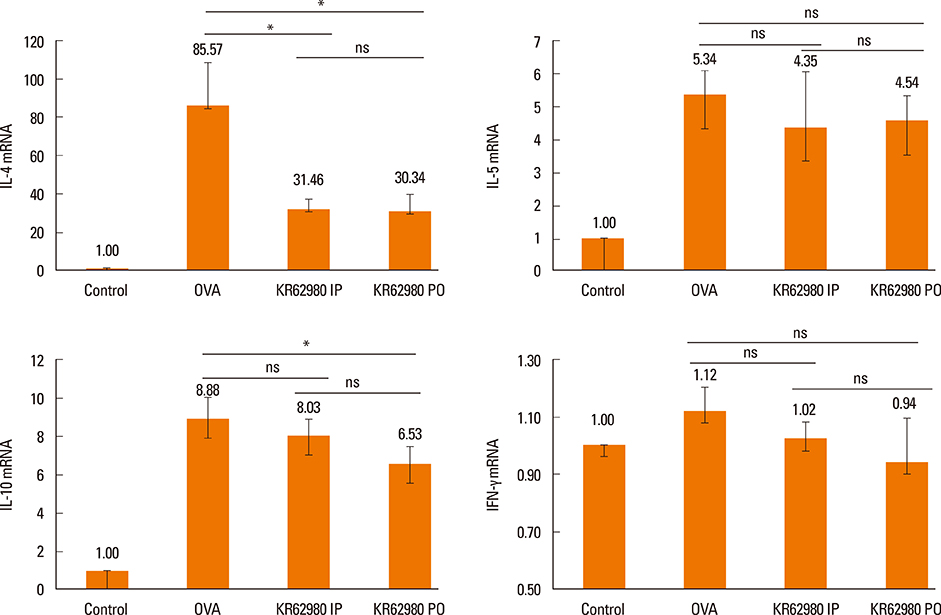
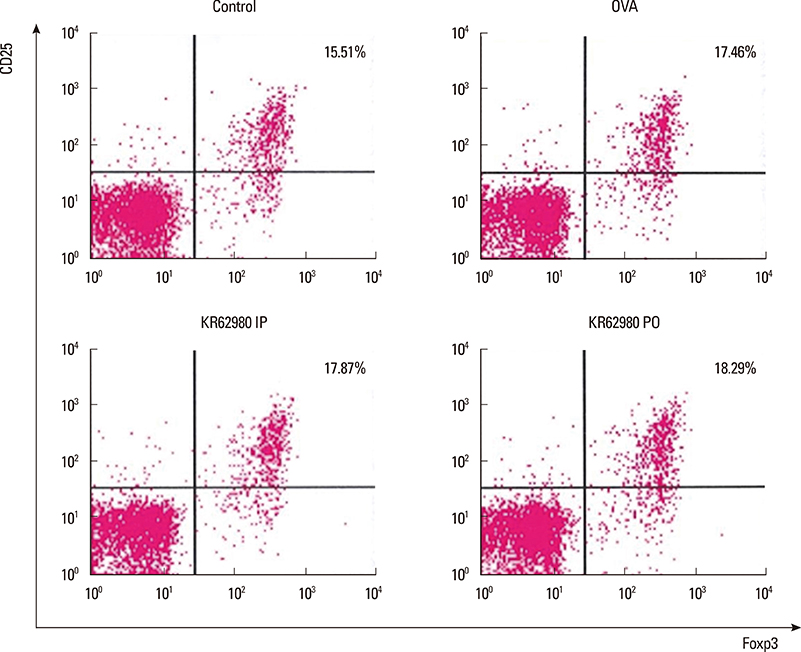
Administration of KR62980 significantly decreased the number of nasal rubbing, nasal sneezing, ova-specific IgE and total IgE in serum, secretion of Interleukin (IL)-4, IL-5, and IL-17 from the spleen and eosinophilic infiltration in the nasal mucosa. KR62980 decreased the expression of IL-4, IL-5 and IL-10 mRNAs in the nasal mucosal tissue, while, it elevated the level of IL-10 and IFN-gamma in splenocyte culture. KR62980 seemed to decrease IL-17 level in local and systemic level even though it did not reach to statistical significance. The anti-inflammatory effect was more definite when the KR62980 was administered intraorally than intraperitoneally.
CONCLUSIONS
A novel PPAR-gamma ligand, KR62980 can attenuate OVA-induced allergic inflammation in mice mainly through modulation of Th2 cytokines. This finding suggests that PPAR-gamma might have a role in the treatment of allergic rhinitis.
MeSH Terms
-
Animals
Cytokines
Eosinophils
Immunoglobulin E
Inflammation
Interleukin-10
Interleukin-17
Interleukin-4
Interleukin-5
Interleukins
Ligands
Macrophages
Mast Cells
Mice*
Mucous Membrane
Nasal Mucosa
Ovalbumin
Ovum
Peroxisomes*
PPAR gamma
Rhinitis*
RNA, Messenger
Sneezing
Spleen
T-Lymphocytes
Cytokines
Immunoglobulin E
Interleukin-10
Interleukin-17
Interleukin-4
Interleukin-5
Interleukins
Ligands
Ovalbumin
PPAR gamma
RNA, Messenger
Figure
Cited by 3 articles
-
Effect of Proparacaine in a Mouse Model of Allergic Rhinitis
Hwan Soo Kim, Sulmui Won, Eu Kyoung Lee, Yoon Hong Chun, Jong-Seo Yoon, Jin Tack Kim, Hyun Hee Kim
Clin Exp Otorhinolaryngol. 2017;10(4):325-331. doi: 10.21053/ceo.2017.00101.Antiallergic Effect of
Hizikia fusiformis in an Ovalbumin-Induced Allergic Rhinitis Mouse Model
Yu-Lian Zhang, Hyun-Jae Shin, Jung-Heon Lee, Jieun Lee
Clin Exp Otorhinolaryngol. 2019;12(2):196-205. doi: 10.21053/ceo.2019.00094.Strain-Specific Differences in House Dust Mite (
Dermatophagoides farinae
Ki-Il Lee, Jun-Sang Bae, Eun Hee Kim, Ji Hye Kim, Lele Lyu, Young-Jun Chung, Ji-Hun Mo
Clin Exp Otorhinolaryngol. 2020;13(4):396-406. doi: 10.21053/ceo.2019.01837.
Reference
-
1. Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988; 240:889–895.2. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001; 19:683–765.3. Oberfield JL, Collins JL, Holmes CP, Goreham DM, Cooper JP, Cobb JE, et al. A peroxisome proliferator-activated receptor gamma ligand inhibits adipocyte differentiation. Proc Natl Acad Sci U S A. 1999; 96:6102–6106.4. Rocchi S, Picard F, Vamecq J, Gelman L, Potier N, Zeyer D, et al. A unique PPARgamma ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol Cell. 2001; 8:737–747.5. Won HY, Min HJ, Ahn JH, Yoo SE, Bae MA, Hong JH, et al. Anti-allergic function and regulatory mechanisms of KR62980 in allergen-induced airway inflammation. Biochem Pharmacol. 2010; 79:888–896.6. Maggi E. The TH1/TH2 paradigm in allergy. Immunotechnology. 1998; 3:233–244.7. Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedón JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005; 5:161–166.8. Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001; 108:430–438.9. Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003; 111:1293–1298.10. Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001; 125:177–183.11. Quan SH, Zhang YL, Han DH, Iwakura Y, Rhee CS. Contribution of interleukin 17A to the development and regulation of allergic inflammation in a murine allergic rhinitis model. Ann Allergy Asthma Immunol. 2012; 108:342–350.12. Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, et al. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005; 175:4255–4264.13. Ueki S, Matsuwaki Y, Kayaba H, Oyamada H, Kanda A, Usami A, et al. Peroxisome proliferator-activated receptor gamma regulates eosinophil functions: a new therapeutic target for allergic airway inflammation. Int Arch Allergy Immunol. 2004; 134:Suppl 1. 30–36.14. Serhan CN, Devchand PR. Novel antiinflammatory targets for asthma. A role for PPARgamma? Am J Respir Cell Mol Biol. 2001; 24:658–661.15. Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin. Clin Immunol. 2005; 114:100–109.16. Park SJ, Lee YC. Peroxisome proliferator-activated receptor gamma as a novel therapeutic target in asthma. J Asthma. 2008; 45:1–8.17. Lee KS, Park SJ, Kim SR, Min KH, Jin SM, Lee HK, et al. Modulation of airway remodeling and airway inflammation by peroxisome proliferator-activated receptor gamma in a murine model of toluene diisocyanate-induced asthma. J Immunol. 2006; 177:5248–5257.18. Kim SR, Lee KS, Park HS, Park SJ, Min KH, Jin SM, et al. Involvement of IL-10 in peroxisome proliferator-activated receptor gamma-mediated anti-inflammatory response in asthma. Mol Pharmacol. 2005; 68:1568–1575.19. Kim KR, Lee JH, Kim SJ, Rhee SD, Jung WH, Yang SD, et al. KR-62980: a novel peroxisome proliferator-activated receptor gamma agonist with weak adipogenic effects. Biochem Pharmacol. 2006; 72:446–454.20. Scheen AJ. Combined thiazolidinedione-insulin therapy: should we be concerned about safety. Drug Saf. 2004; 27:841–856.21. Martens FM, Visseren FL, Lemay J, de Koning EJ, Rabelink TJ. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002; 62:1463–1480.22. Chui PC, Guan HP, Lehrke M, Lazar MA. PPARgamma regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J Clin Invest. 2005; 115:2244–2256.23. Takanaski S, Nonaka R, Xing Z, O’Byrne P, Dolovich J, Jordana M. Interleukin 10 inhibits lipopolysaccharide-induced survival and cytokine production by human peripheral blood eosinophils. J Exp Med. 1994; 180:711–715.24. Baumann R, Rabaszowski M, Stenin I, Tilgner L, Scheckenbach K, Wiltfang J, et al. Comparison of the nasal release of IL-4, IL-10, IL-17, CCL13/MCP-4, and CCL26/eotaxin-3 in allergic rhinitis during season and after allergen challenge. Am J Rhinol Allergy. 2013; 27:266–272.25. Park SJ, Lee KS, Kim SR, Min KH, Choe YH, Moon H, et al. Peroxisome proliferator-activated receptor gamma agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J Immunol. 2009; 183:3259–3267.26. Housley WJ, O’Conor CA, Nichols F, Puddington L, Lingenheld EG, Zhu L, et al. PPARgamma regulates retinoic acid-mediated DC induction of Tregs. J Leukoc Biol. 2009; 86:293–301.27. Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanisms. J Immunol. 2007; 178:4129–4135.28. Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, et al. Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol. 2007; 178:2122–2131.29. Wang W, Zhu Z, Zhu B, Ma Z. Peroxisome proliferator-activated receptor-gamma agonist induces regulatory T cells in a murine model of allergic rhinitis. Otolaryngol Head Neck Surg. 2011; 144:506–513.30. Cheng X, Lou W, Wang C, Zhang W, Han D, Zhang L. FOXP3-marked IL-17a-producing regulatory T cells are increased in patients with allergic rhinitis. Acta Otolaryngol. 2012; 132:1311–1317.31. Park JS, Kim MS, Song JS, Choi SH, Lee BH, Woo J, et al. Dose-independent pharmacokinetics of a new peroxisome proliferator-activated receptor-γ agonist, KR-62980, in Sprague-Dawley rats and ICR mice. Arch Pharm Res. 2011; 34:2051–2058.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allergic Rhinitis Mouse Model
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy
- Peroxisome Proliferator-Activated Receptor (PPAR) alpha/gamma Agonist
- Effects of Sulfonylureas on Peroxisome Proliferator-Activated Receptor gamma Activity and on Glucose Uptake by Thiazolidinediones
- Refocusing Peroxisome Proliferator Activated Receptor-alpha: A New Insight for Therapeutic Roles in Diabetes