Diabetes Metab J.
2011 Aug;35(4):340-347. 10.4093/dmj.2011.35.4.340.
Effects of Sulfonylureas on Peroxisome Proliferator-Activated Receptor gamma Activity and on Glucose Uptake by Thiazolidinediones
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. kspark@snu.ac.kr
- KMID: 2281640
- DOI: http://doi.org/10.4093/dmj.2011.35.4.340
Abstract
- BACKGROUND
Sulfonylurea primarily stimulates insulin secretion by binding to its receptor on the pancreatic beta-cells. Recent studies have suggested that sulfonylureas induce insulin sensitivity through peroxisome proliferator-activated receptor gamma (PPARgamma), one of the nuclear receptors. In this study, we investigated the effects of sulfonylurea on PPARgamma transcriptional activity and on the glucose uptake via PPARgamma.
METHODS
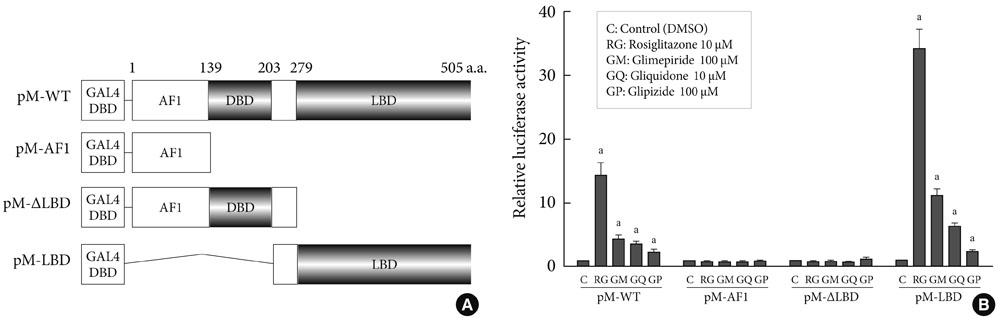
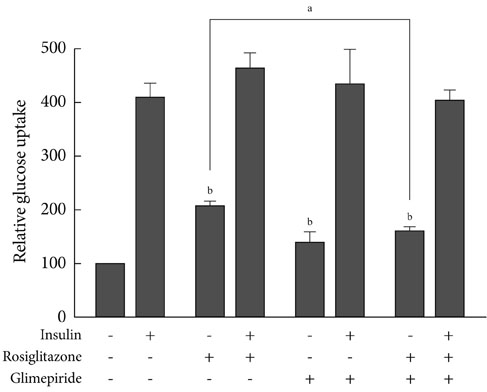
Transcription reporter assays using Cos7 cells were performed to determine if specific sulfonylureas stimulate PPARgamma transactivation. Glimepiride, gliquidone, and glipizide (1 to 500 microM) were used as treatment, and rosiglitazone at 1 and 10 microM was used as a control. The effects of sulfonylurea and rosiglitazone treatments on the transcriptional activity of endogenous PPARgamma were observed. In addition, 3T3-L1 adipocytes were treated with rosiglitazone (10 microM), glimepiride (100 microM) or both to verify the effect of glimepiride on rosiglitazone-induced glucose uptake.
RESULTS
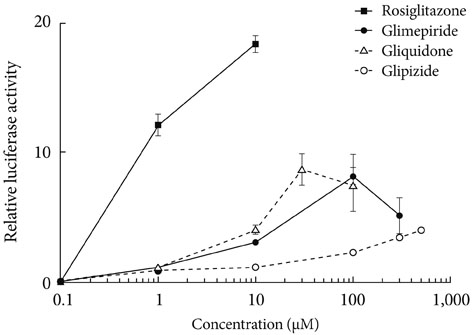
Sulfonylureas, including glimepiride, gliquidone and glipizide, increased PPARgamma transcriptional activity, gliquidone being the most potent PPARgamma agonist. However, no additive effects were observed in the presence of rosiglitazone. When rosiglitazone was co-treated with glimepiride, PPARgamma transcriptional activity and glucose uptake were reduced compared to those after treatment with rosiglitazone alone. This competitive effect of glimepiride was observed only at high concentrations that are not achieved with clinical doses.
CONCLUSION
Sulfonylureas like glimepiride, gliquidone and glipizide increased the transcriptional activity of PPARgamma. Also, glimepiride was able to reduce the effect of rosiglitazone on PPARgamma agonistic activity and glucose uptake. However, the competitive effect does not seem to occur at clinically feasible concentrations.
Keyword
MeSH Terms
-
Adipocytes
Diabetes Mellitus, Type 2
Glipizide
Glucose
Insulin
Insulin Resistance
Peroxisome Proliferator-Activated Receptors
Peroxisomes
PPAR gamma
Receptors, Cytoplasmic and Nuclear
Sulfonylurea Compounds
Thiazolidinediones
Transcriptional Activation
Glipizide
Glucose
Insulin
PPAR gamma
Peroxisome Proliferator-Activated Receptors
Receptors, Cytoplasmic and Nuclear
Sulfonylurea Compounds
Thiazolidinediones
Figure
Reference
-
1. Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.2. Taylor SI. Deconstructing type 2 diabetes. Cell. 1999. 97:9–12.3. Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J Clin Invest. 2000. 106:467–472.4. Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008. 77:289–312.5. Panigrahy D, Singer S, Shen LQ, Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing S, Fletcher C, Fletcher JA, Hlatky L, Hahnfeldt P, Folkman J, Kaipainen A. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest. 2002. 110:923–932.6. Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992. 355:446–449.7. Renaud JP, Moras D. Structural studies on nuclear receptors. Cell Mol Life Sci. 2000. 57:1748–1769.8. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999. 20:649–688.9. Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998. 93:229–240.10. Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005. 102:2340–2345.11. Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005. 123:993–999.12. Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997. 272:3406–3410.13. Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004. 43:993–1002.14. Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004. 109:2054–2057.15. Fukuen S, Iwaki M, Yasui A, Makishima M, Matsuda M, Shimomura I. Sulfonylurea agents exhibit peroxisome proliferator-activated receptor gamma agonistic activity. J Biol Chem. 2005. 280:23653–23659.16. Inukai K, Watanabe M, Nakashima Y, Takata N, Isoyama A, Sawa T, Kurihara S, Awata T, Katayama S. Glimepiride enhances intrinsic peroxisome proliferator-activated receptor-gamma activity in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005. 328:484–490.17. Arrault A, Rocchi S, Picard F, Maurois P, Pirotte B, Vamecq J. A short series of antidiabetic sulfonylureas exhibit multiple ligand PPARgamma-binding patterns. Biomed Pharmacother. 2009. 63:56–62.18. Scarsi M, Podvinec M, Roth A, Hug H, Kersten S, Albrecht H, Schwede T, Meyer UA, Rucker C. Sulfonylureas and glinides exhibit peroxisome proliferator-activated receptor gamma activity: a combined virtual screening and biological assay approach. Mol Pharmacol. 2007. 71:398–406.19. Langtry HD, Balfour JA. Glimepiride: a review of its use in the management of type 2 diabetes mellitus. Drugs. 1998. 55:563–584.20. Malerczyk V, Badian M, Korn A, Lehr KH, Waldhausl W. Dose linearity assessment of glimepiride (Amaryl) tablets in healthy volunteers. Drug Metabol Drug Interact. 1994. 11:341–357.21. von Nicolai H, Brickl R, Eschey H, Greischel A, Heinzel G, Konig E, Limmer J, Rupprecht E. Duration of action and pharmacokinetics of the oral antidiabetic drug gliquidone in patients with non-insulin-dependent (type 2) diabetes mellitus. Arzneimittelforschung. 1997. 47:247–252.22. Inukai K, Watanabe M, Nakashima Y, Sawa T, Takata N, Tanaka M, Kashiwabara H, Yokota K, Suzuki M, Kurihara S, Awata T, Katayama S. Efficacy of glimepiride in Japanese type 2 diabetic subjects. Diabetes Res Clin Pract. 2005. 68:250–257.23. Jaber LA, Ducharme MP, Edwards DJ, Slaughter RL, Grunberger G. The influence of multiple dosing and age on the pharmacokinetics and pharmacodynamics of glipizide in patients with type II diabetes mellitus. Pharmacotherapy. 1996. 16:760–768.24. Hiromori Y, Nishikawa J, Yoshida I, Nagase H, Nakanishi T. Structure-dependent activation of peroxisome proliferator-activated receptor (PPAR) gamma by organotin compounds. Chem Biol Interact. 2009. 180:238–244.25. Mori RC, Hirabara SM, Hirata AE, Okamoto MM, Machado UF. Glimepiride as insulin sensitizer: increased liver and muscle responses to insulin. Diabetes Obes Metab. 2008. 10:596–600.26. Rosskamp R, Wernicke-Panten K, Draeger E. Clinical profile of the novel sulphonylurea glimepiride. Diabetes Res Clin Pract. 1996. 31:Suppl. S33–S42.27. Haupt A, Kausch C, Dahl D, Bachmann O, Stumvoll M, Haring HU, Matthaei S. Effect of glimepiride on insulin-stimulated glycogen synthesis in cultured human skeletal muscle cells: a comparison to glibenclamide. Diabetes Care. 2002. 25:2129–2132.28. Muller G, Wied S, Wetekam EM, Crecelius A, Unkelbach A, Punter J. Stimulation of glucose utilization in 3T3 adipocytes and rat diaphragm in vitro by the sulphonylureas, glimepiride and glibenclamide, is correlated with modulations of the cAMP regulatory cascade. Biochem Pharmacol. 1994. 48:985–996.29. Muller G. The molecular mechanism of the insulin-mimetic/sensitizing activity of the antidiabetic sulfonylurea drug Amaryl. Mol Med. 2000. 6:907–933.30. Fernyhough ME, Okine E, Hausman G, Vierck JL, Dodson MV. PPARgamma and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest Anim Endocrinol. 2007. 33:367–378.31. Ezaki O. Regulatory elements in the insulin-responsive glucose transporter (GLUT4) gene. Biochem Biophys Res Commun. 1997. 241:1–6.32. McGowan KM, Long SD, Pekala PH. Glucose transporter gene expression: regulation of transcription and mRNA stability. Pharmacol Ther. 1995. 66:465–505.33. Hamm JK, el Jack AK, Pilch PF, Farmer SR. Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann N Y Acad Sci. 1999. 892:134–145.34. Kahn BB, McGraw TE. Rosiglitazone, PPARγ, and type 2 diabetes. N Engl J Med. 2010. 363:2667–2669.35. Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A, Elliott P. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009. 339:b4731.36. Evans JM, Ogston SA, Emslie-Smith A, Morris AD. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia. 2006. 49:930–936.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Co-treatment with Dexamethasone and Troglitazone on Transcription and Secretion of IL-8 in the A549 Airway Epithelial Cells
- Targeting the Peroxisome Proliferator-Activated Receptor-gamma to Counter the Inflammatory Milieu in Obesity
- Peroxisome Proliferator-Activated Receptor (PPAR) alpha/gamma Agonist
- Role of Pexoxisome Proliferator Activated Receptor Gamma in Growth Regulation of Thyroid Cancer Cells
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy