Korean Diabetes J.
2009 Oct;33(5):375-381. 10.4093/kdj.2009.33.5.375.
The Role of Hypothalamic FoxO1 on Hyperphagia in Streptozotocin-Induced Diabetic Mice
- Affiliations
-
- 1Department of Internal Medicine, University of Ulsan College of Medicine, Ulsan University Hospital, Ulsan, Korea. yikuls@uuh.ulsan.kr
- 2Biomedical Research Center, University of Ulsan College of Medicine, Ulsan, Korea.
- 3Department of Biological Sciences, University of Ulsan, Ulsan, Korea.
- KMID: 2231144
- DOI: http://doi.org/10.4093/kdj.2009.33.5.375
Abstract
- BACKGROUND
Streptozotocin-induced diabetic animals are characterized by hyperphagia due to deficiencies of insulin and leptin. Forkhead box-containing protein of the O subfamily-1 (FoxO1) regulates energy homeostasis by regulating energy expenditure and food intake as well as mediating insulin and leptin signals in the hypothalamus. To identify the mediator of diabetic hyperphagia, we examined the effects of insulin or leptin on hypothalamic FoxO1 expression in a diabetic animal model.
METHODS
Diabetes was induced in mice (C57BL/6) by intraperitoneal administration of streptozotocin (200 mg/kg). Stainless steel cannula was implanted into the lateral ventricle of the brain in each mouse. After three weeks, the mice were administered saline, insulin or leptin via intracerebroventricular (ICV) route. The medial hypothalamus was isolated to evaluate the mRNA expressions of FoxO1 and neuropeptides.
RESULTS
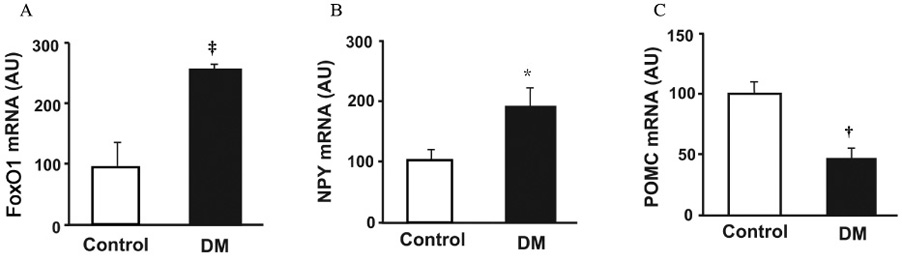
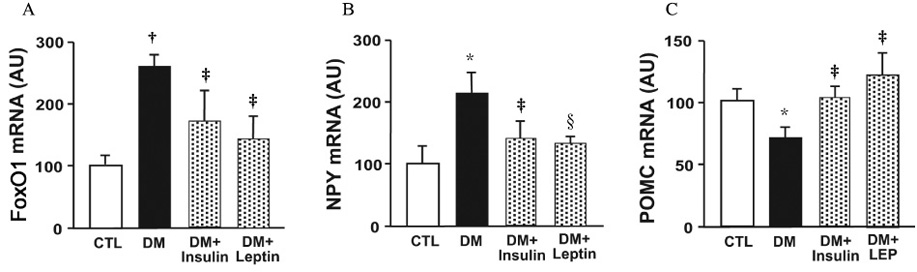
Streptozotocin-induced diabetic mice exhibited significant elevations of blood glucose and food intake and significantly low levels of serum insulin and leptin. The levels of hypothalamic FoxO1 mRNA were significantly increased in diabetic mice. The hypothalamic expression of neuropeptide Y (NPY) mRNA was increased, but the expression of preproopiomelanocortin (POMC) mRNA was decreased in diabetic mice. ICV administration of insulin or leptin attenuated the upregulation of hypothalamic FoxO1 mRNA, and resulted in downregulation of NPY mRNA and upregulation of POMC mRNA in diabetic mice.
CONCLUSION
We observed that the expression of hypothalamic FoxO1 mRNA was increased in streptozotocin-induced diabetic mice, and that it was significantly attenuated by central administration of insulin or leptin. These results suggest that hypothalamic FoxO1 is the direct mediator of diabetic hyperphagia.
Keyword
MeSH Terms
-
Animals
Blood Glucose
Brain
Catheters
Diabetes Mellitus
Down-Regulation
Eating
Energy Metabolism
Forkhead Transcription Factors
Homeostasis
Hyperphagia
Hypothalamus
Hypothalamus, Middle
Insulin
Lateral Ventricles
Leptin
Mice
Negotiating
Neuropeptide Y
Pro-Opiomelanocortin
RNA, Messenger
Stainless Steel
Streptozocin
Up-Regulation
Blood Glucose
Forkhead Transcription Factors
Insulin
Leptin
Neuropeptide Y
Pro-Opiomelanocortin
RNA, Messenger
Stainless Steel
Streptozocin
Figure
Reference
-
1. Booth DA. Some characteristics of feeding during streptozotocin-induced diabetes in the rat. J Comp Physiol Psychol. 1972. 80:238–249.2. Hidaka S, Yoshimatsu H, Kondou S, Oka K, Tsuruta Y, Sakino H, Itateyama E, Noguchi H, Himeno K, Okamoto K, Teshima Y, Okeda T, Sakata T. Hypoleptinemia, but not hypoinsulinemia, induces hyperphagia in streptozotocin-induced diabetic rats. J Neurochem. 2001. 77:993–1000.3. Namkoong C, Kim MS, Jang PG, Han SM, Park HS, Koh EH, Lee WJ, Kim JY, Park IS, Park JY, Lee KU. Enhanced hypothalamic AMP-activated protein kinase activity contributes to hyperphagia in diabetic rats. Diabetes. 2005. 54:63–68.4. Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahrén B. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998. 274:1482–1491.5. Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995. 44:147–151.6. Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes. 1999. 48:1275–1280.7. Sindelar DK, Mystkowski P, Marsh DJ, Palmiter RD, Schwartz MW. Attenuation of diabetic hyperphagia in neuropeptide Y--deficient mice. Diabetes. 2002. 51:778–783.8. Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006. 9:901–906.9. Kitamura T, Feng Y, Kitamura YI, Chua SC Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006. 12:534–540.10. Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008. 27:2320–2336.11. Friedman JM. Modern science versus the stigma of obesity. Nat Med. 2004. 10:563–569.12. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006. 443:289–295.13. Williams G, Steel JH, Cardoso H, Ghatei MA, Lee YC, Gill JS, Burrin JM, Polak JM, Bloom SR. Increased hypothalamic neuropeptide Y concentrations in diabetic rat. Diabetes. 1988. 37:763–772.14. Nakae J, Kitamura T, Kitamura Y, Biggs WH 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003. 4:119–129.15. Buteau J, Accili D. Regulation of pancreatic beta-cell function by the forkhead protein FoxO1. Diabetes Obes Metab. 2007. 9:suppl 2. S140–S146.16. Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008. 8:65–76.17. Ropelle ER, Pauli JR, Prada P, Cintra DE, Rocha GZ, Moraes JC, Frederico MJ, da Luz G, Pinho RA, Carvalheira JB, Velloso LA, Saad MA, De Souza CT. Inhibition of hypothalamic Foxo1 expression reduced food intake in diet-induced obesity rats. J Physiol. 2009. 587:2341–2351.18. Gelling RW. Diabetic hyperphagia--ghrelin in the driver's seat. Endocrinology. 2006. 147:2631–2633.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypothalamic AMPK Activity in Diabetic Rats
- Apoptosis and Ultrastructural Changes of Glomerular Endothelial Cells of Mice with Streptozotocin-induced Diabetic Nephropathy
- MiR-542-5p Inhibits Hyperglycemia and Hyperlipoidemia by Targeting FOXO1 in the Liver
- Mechanism of Lipid Accumulation through PAR2 Signaling in Diabetic Male Mice
- ATP-Sensitive Potassium Channel-Deficient Mice Show Hyperphagia but Are Resistant to Obesity