Endocrinol Metab.
2021 Feb;36(1):171-184. 10.3803/EnM.2020.850.
Mechanism of Lipid Accumulation through PAR2 Signaling in Diabetic Male Mice
- Affiliations
-
- 1Department of Pharmacy, College of Pharmacy, Pusan National University, Busan, Korea
- KMID: 2513300
- DOI: http://doi.org/10.3803/EnM.2020.850
Abstract
- Background
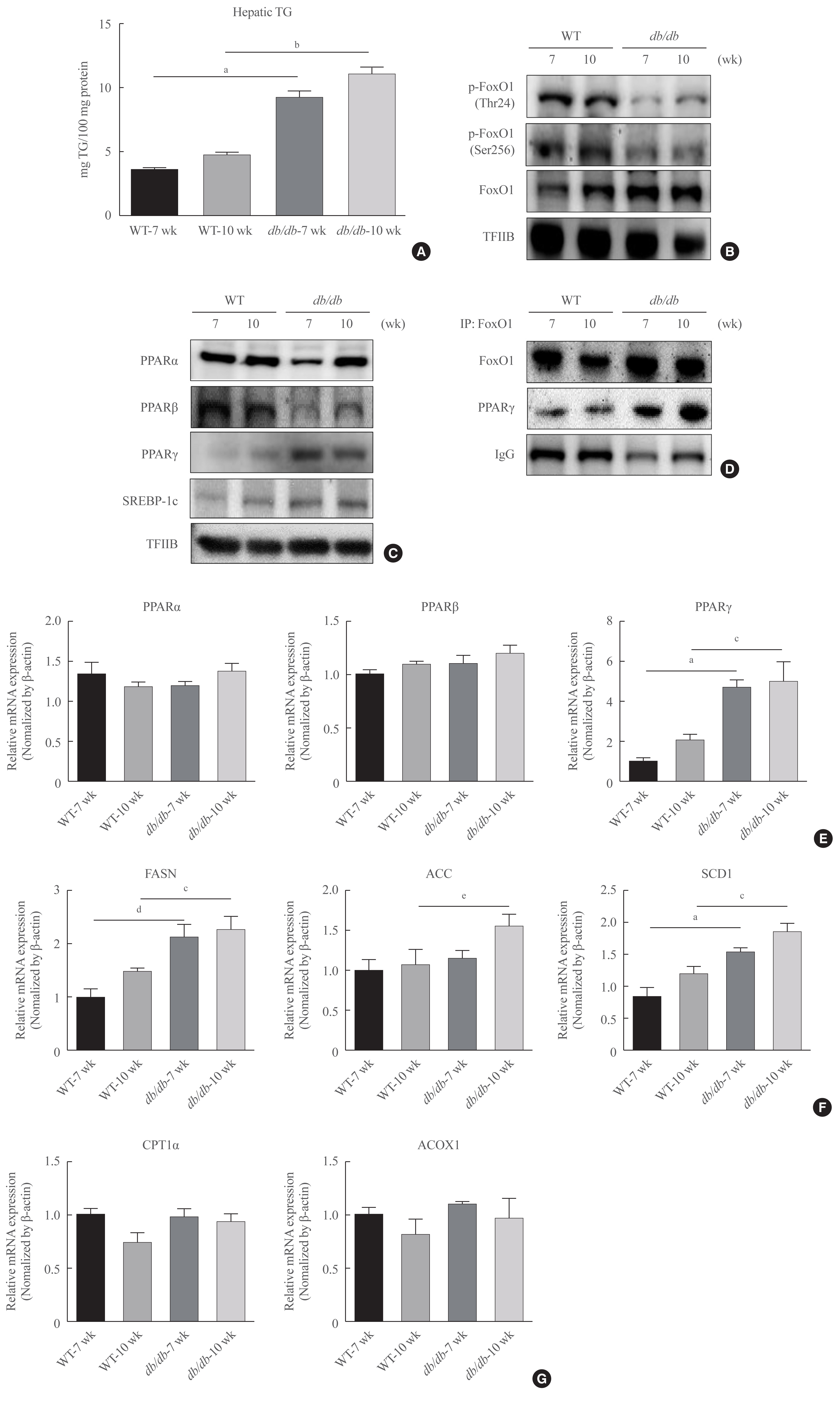
Protease-activated protein-2 (PAR2) has been reported to regulate hepatic insulin resistance condition in type 2 diabetes mice. However, the mechanism of lipid metabolism through PAR2 in obesity mice have not yet been examined. In liver, Forkhead box O1 (FoxO1) activity induces peroxisome proliferator-activated receptor γ (PPARγ), leading to accumulation of lipids and hyperlipidemia. Hyperlipidemia significantly influence hepatic steatoses, but the mechanisms underlying PAR2 signaling are complex and have not yet been elucidated.
Methods
To examine the modulatory action of FoxO1 and its altered interaction with PPARγ, we utilized db/db mice and PAR2-knockout (KO) mice administered with high-fat diet (HFD).
Results
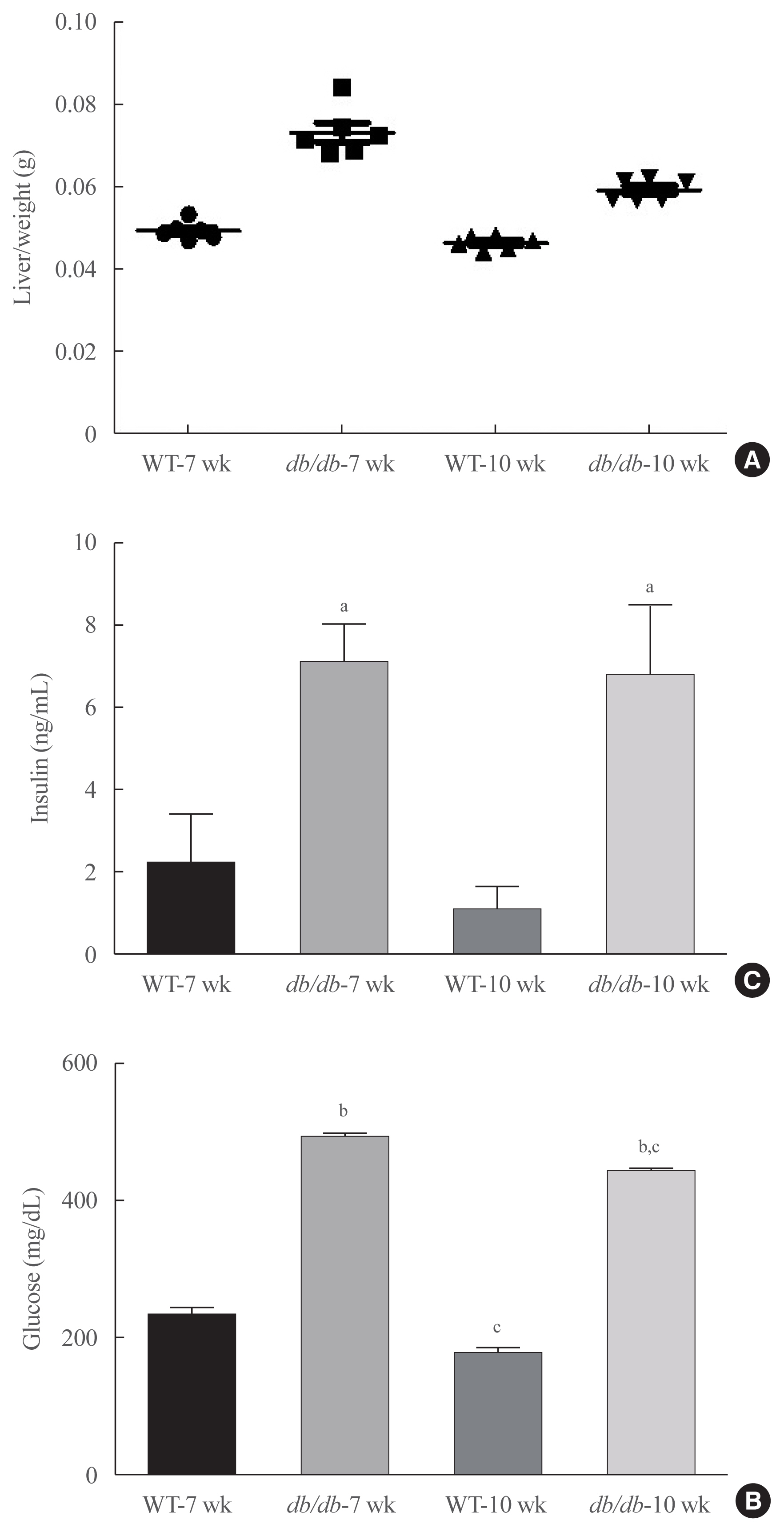
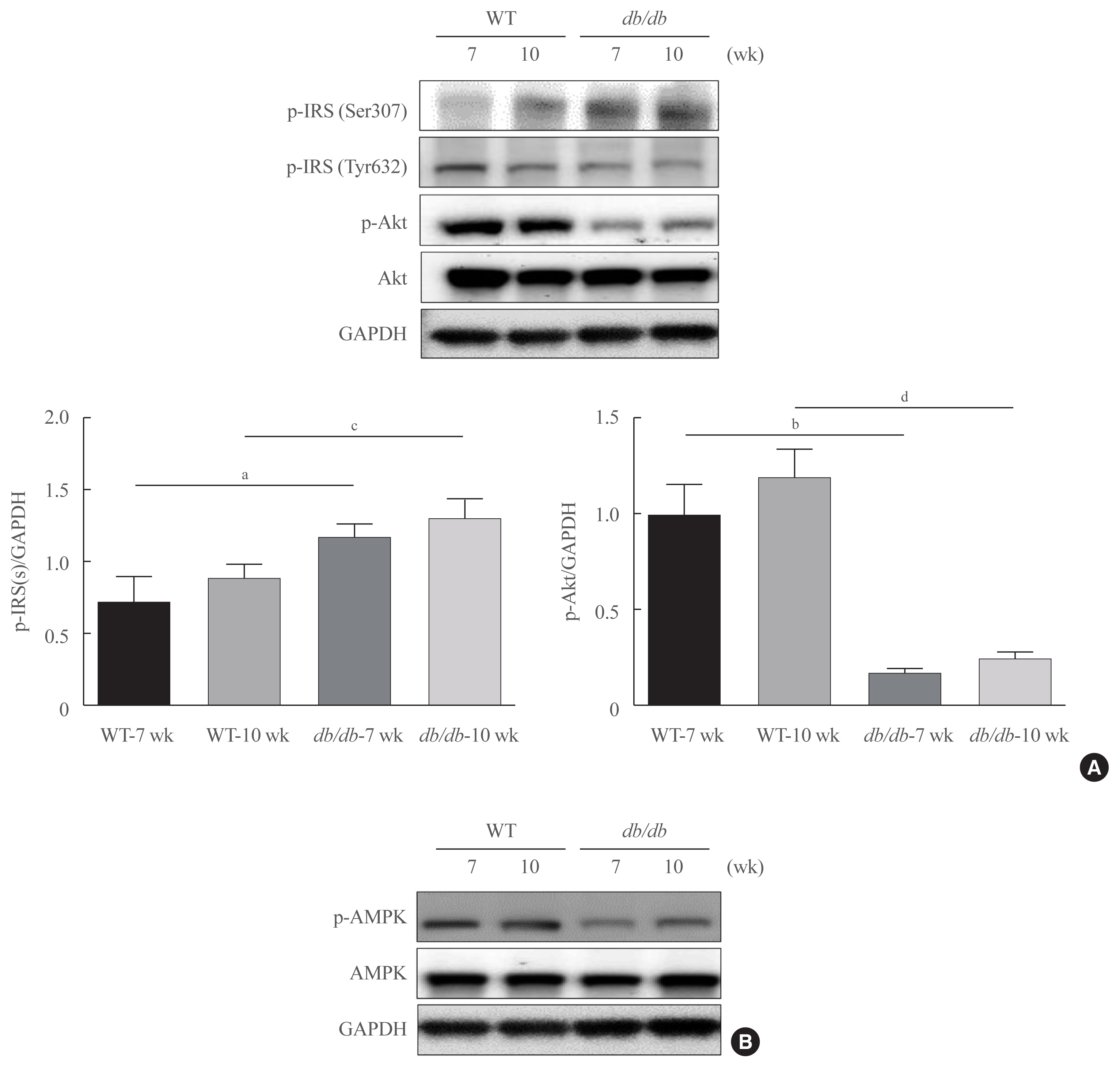
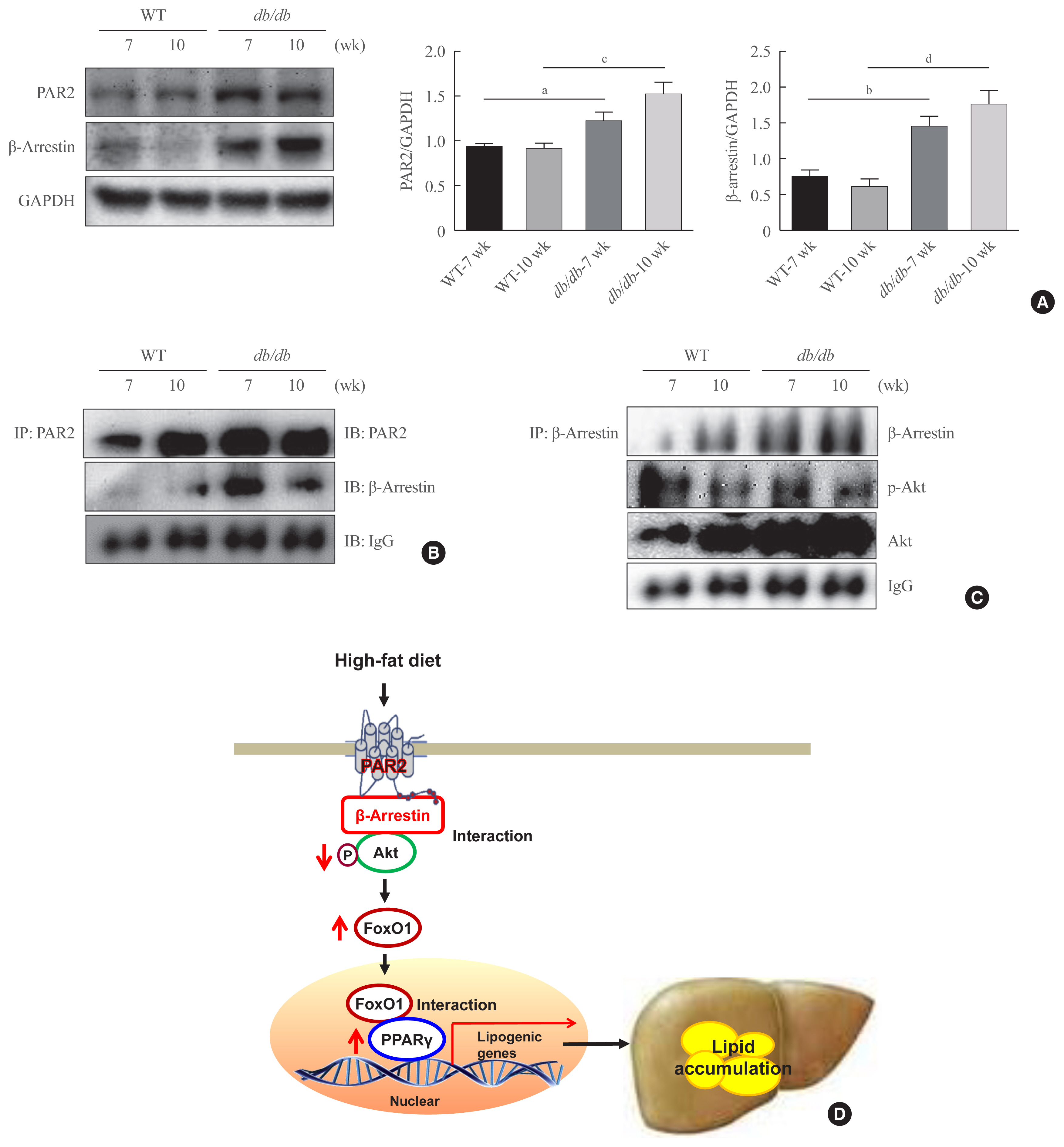
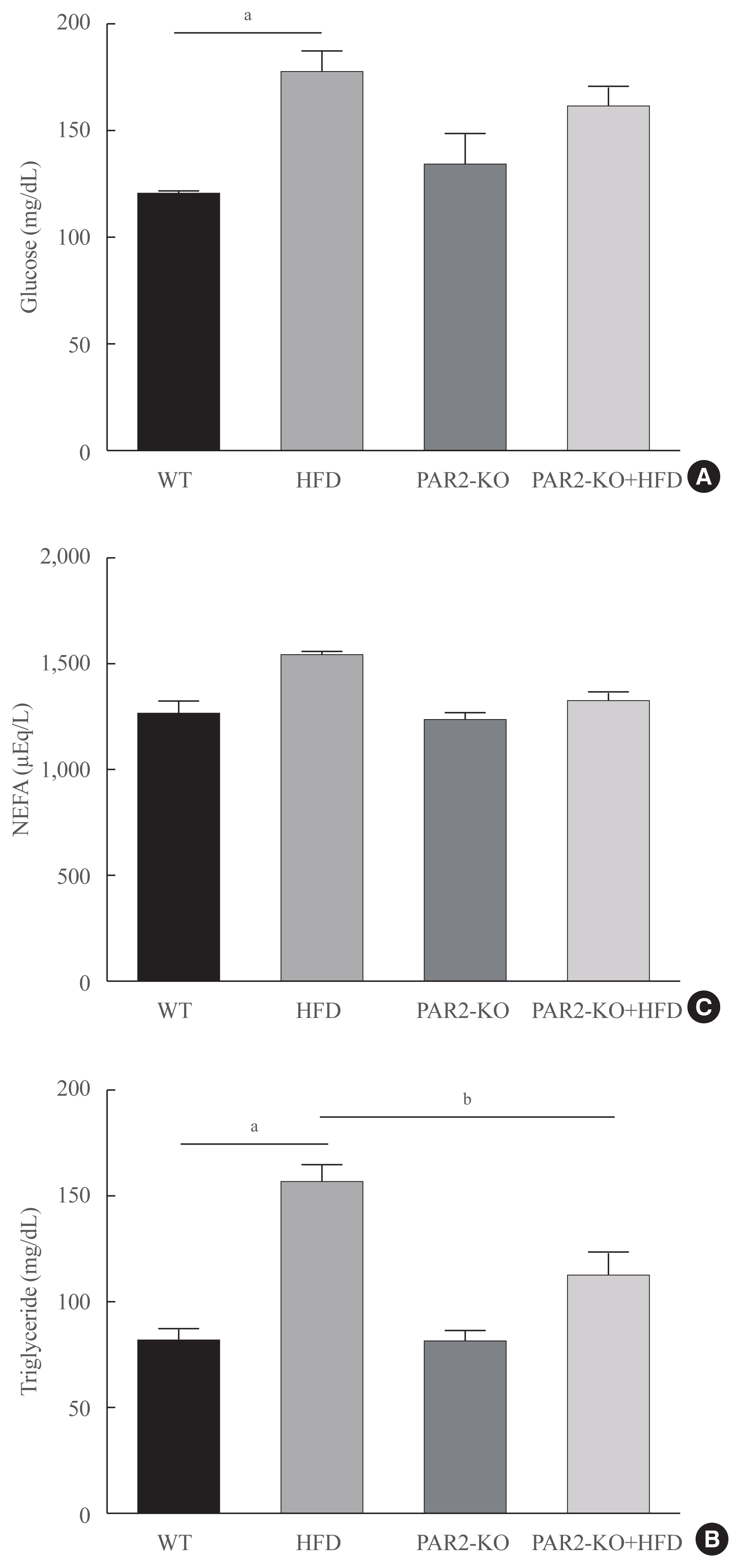
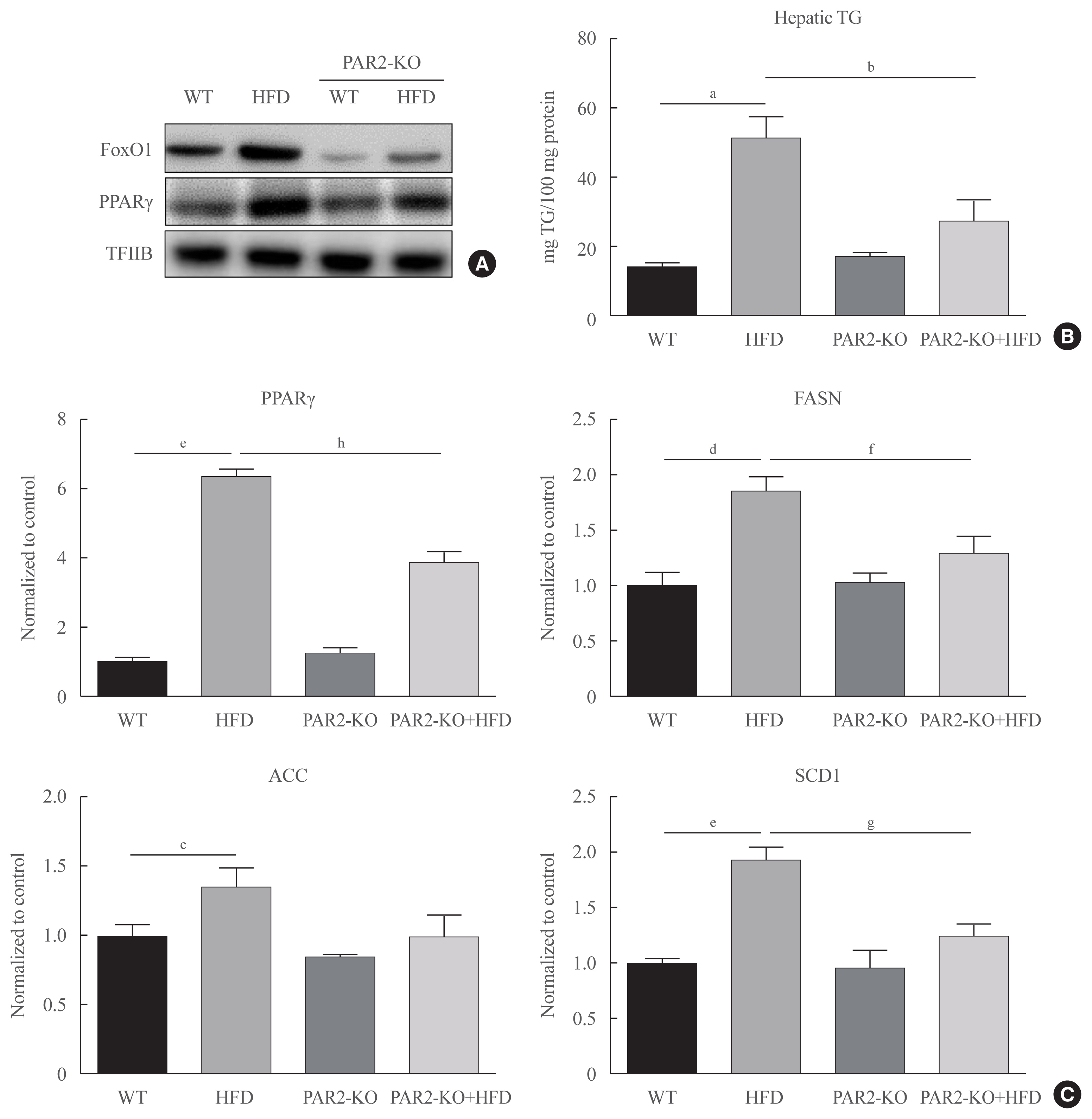
Here, we demonstrated that PAR2 was overexpressed and regulated downstream gene expressions in db/db but not in db+ mice. The interaction between PAR2/β-arrestin and Akt was also greater in db/db mice. The Akt inhibition increased FoxO1 activity and subsequently PPARγ gene in the livers that led to hepatic lipid accumulation. Our data showed that FoxO1 was negatively controlled by Akt signaling and consequently, the activity of a major lipogenesis-associated transcription factors such as PPARγ increased, leading to hepatic lipid accumulation through the PAR2 pathway under hyperglycemic conditions in mice. Furthermore, the association between PPARγ and FoxO1 was increased in hepatic steatosis condition in db/db mice. However, HFD-fed PAR2-KO mice showed suppressed FoxO1-induced hepatic lipid accumulation compared with HFD-fed control groups.
Conclusion
Collectively, our results provide evidence that the interaction of FoxO1 with PPARγ promotes hepatic steatosis in mice. This might be due to defects in PAR2/β-arrestin-mediated Akt signaling in diabetic and HFD-fed mice.
Figure
Reference
-
1. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014; 15:6184–223.
Article2. Groneberg DA, Franke K, Klingelhofer D, Schwarzer M, Ohlendorf D. Density equalizing mapping of obesity: analysis of a global epidemic. Z Gastroenterol. 2015; 53:553–61.
Article3. Newell-Fugate AE. The role of sex steroids in white adipose tissue adipocyte function. Reproduction. 2017; 153:R133–49.
Article4. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015; 402:113–9.
Article5. Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004; 117:421–6.
Article6. Karger S, Weidinger C, Krause K, Sheu SY, Aigner T, Gimm O, et al. FOXO3a: a novel player in thyroid carcinogenesis? Endocr Relat Cancer. 2009; 16:189–99.
Article7. Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005; 16:183–9.
Article8. Biggs WH 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999; 96:7421–6.
Article9. Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, et al. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006; 281:1091–8.
Article10. Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008; 57:846–59.11. Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004; 380(Pt 2):297–309.
Article12. Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005; 123:993–9.13. Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, et al. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008; 7:302–11.14. Wierzbicki M, Chabowski A, Zendzian-Piotrowska M, Gorski J. Differential effects of in vivo PPAR alpha and gamma activation on fatty acid transport proteins expression and lipid content in rat liver. J Physiol Pharmacol. 2009; 60:99–106.15. Kim DH, Zhang T, Lee S, Calabuig-Navarro V, Yamauchi J, Piccirillo A, et al. FoxO6 integrates insulin signaling with MTP for regulating VLDL production in the liver. Endocrinology. 2014; 155:1255–67.
Article16. Bose SK, Kim H, Meyer K, Wolins N, Davidson NO, Ray R. Forkhead box transcription factor regulation and lipid accumulation by hepatitis C virus. J Virol. 2014; 88:4195–203.
Article17. Polvani S, Tarocchi M, Galli A. PPARγ and oxidative stress: con(β) catenating NRF2 and FOXO. PPAR Res. 2012; 2012:641087.
Article18. Kim DH, Lee B, Kim MJ, Park MH, An HJ, Lee EK, et al. Molecular mechanism of betaine on hepatic lipid metabolism: inhibition of forkhead box O1 (FoxO1) binding to peroxisome proliferator-activated receptor gamma (PPARγ). J Agric Food Chem. 2016; 64:6819–25.
Article19. Haslam DW, James WP. Obesity. Lancet. 2005; 366:1197–209.
Article20. Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011; 14:9–19.
Article21. Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol. 2012; 34:133–49.
Article22. Badeanlou L, Furlan-Freguia C, Yang G, Ruf W, Samad F. Tissue factor-protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation. Nat Med. 2011; 17:1490–7.
Article23. Srinivasan R, Ozhegov E, van den Berg YW, Aronow BJ, Franco RS, Palascak MB, et al. Splice variants of tissue factor promote monocyte-endothelial interactions by triggering the expression of cell adhesion molecules via integrin-mediated signaling. J Thromb Haemost. 2011; 9:2087–96.
Article24. Park MK, Cho MK, Kang SA, Kim BY, Yu HS. The induction of the collagen capsule synthesis by Trichinella spiralis is closely related to protease-activated receptor 2. Vet Parasitol. 2016; 230:56–61.
Article25. Coudriet GM, Delmastro-Greenwood MM, Previte DM, Marre ML, O’Connor EC, Novak EA, et al. Treatment with a catalytic superoxide dismutase (SOD) mimetic improves liver steatosis, insulin sensitivity, and inflammation in obesity-induced type 2 diabetes. Antioxidants (Basel). 2017; 6:85.
Article26. Guo S. Molecular basis of insulin resistance: the role of IRS and foxo1 in the control of diabetes mellitus and its complications. Drug Discov Today Dis Mech. 2013; 10:e27–33.
Article27. Shearer AM, Rana R, Austin K, Baleja JD, Nguyen N, Bohm A, et al. Targeting liver fibrosis with a cell-penetrating protease-activated receptor-2 (PAR2) pepducin. J Biol Chem. 2016; 291:23188–98.
Article28. Kim DH, Lee B, Lee J, Kim ME, Lee JS, Chung JH, et al. FoxO6-mediated IL-1β induces hepatic insulin resistance and age-related inflammation via the TF/PAR2 pathway in aging and diabetic mice. Redox Biol. 2019; 24:101184.
Article29. Qu S, Altomonte J, Perdomo G, He J, Fan Y, Kamagate A, et al. Aberrant forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006; 147:5641–52.
Article30. Cook JR, Matsumoto M, Banks AS, Kitamura T, Tsuchiya K, Accili D. A mutant allele encoding DNA binding-deficient FoxO1 differentially regulates hepatic glucose and lipid metabolism. Diabetes. 2015; 64:1951–65.
Article31. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005; 120:483–95.
Article32. Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010; 24:5073–9.
Article33. Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002; 419:316–21.
Article34. Ables GP. Update on PPARγ and nonalcoholic fatty liver disease. PPAR Res. 2012; 2012:912351.
Article35. Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006; 116:2464–72.
Article36. Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S, et al. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005; 336:215–22.37. Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003; 111:737–47.38. Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003; 278:498–505.
Article39. Navab M, Gharavi N, Watson AD. Inflammation and metabolic disorders. Curr Opin Clin Nutr Metab Care. 2008; 11:459–64.
Article40. Galgani JE, Uauy RD, Aguirre CA, Diaz EO. Effect of the dietary fat quality on insulin sensitivity. Br J Nutr. 2008; 100:471–9.
Article41. Tereshina EV. Metabolic abnormalities as a basis for age-dependent diseases and aging? State of the art. Adv Gerontol. 2009; 22:129–38.42. Noguerol J, Roustan PJ, N’Taye M, Delcombel L, Rolland C, Guiraud L, et al. Sexual dimorphism in PAR2-dependent regulation regulation of primitive colonic cells. Biol Sex Differ. 2019; 10:47.43. Wang J, Chakrabarty S, Bui Q, Ruf W, Samad F. Hematopoietic tissue factor-protease-activated receptor 2 signaling promotes hepatic inflammation and contributes to pathways of gluconeogenesis and steatosis in obese mice. Am J Pathol. 2015; 185:524–35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pear pomace ethanol extract improves insulin resistance through enhancement of insulin signaling pathway without lipid accumulation
- Supplementary Effect of gamma-Oryzanol on Lipid Metabolism in Diabetic KK Mice
- Betulin Targets Lipin1/2-Meidated P2X7 Receptor as a Therapeutic Approach to Attenuate Lipid Accumulation and Metaflammation
- Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy
- The Effect of Rice Germ Oil Supplement on Serum and Hepatic Lipid Levels of Streptozotocin-Induced Diabetic Mice