Korean Diabetes J.
2010 Apr;34(2):71-76. 10.4093/kdj.2010.34.2.71.
Functional and Mechanistic Integration of Infection and the Metabolic Syndrome
- Affiliations
-
- 1Cell Biology of Retroviruses Group, Institut Pasteur Korea, Seongnam, Korea.
- 2Diabetes Group, Institut Pasteur Korea, Seongnam, Korea. gary@ip-korea.org
- 3Department of Biology, York University, Toronto, Canada.
- KMID: 2222379
- DOI: http://doi.org/10.4093/kdj.2010.34.2.71
Abstract
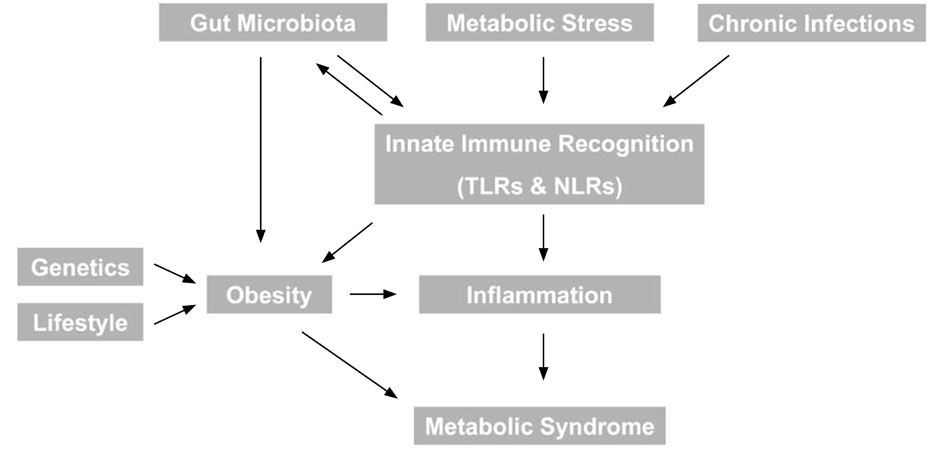
- The metabolic syndrome refers to a well defined group of risk factors, including central obesity and inflammation, for the development of diabetes and cardiovascular disease. Interestingly, many studies have recently led to the emergence of somewhat unexpected relationships between several infectious diseases and various aspects of the metabolic syndrome. Our understanding of the mechanisms underlying these interactions is also rapidly developing and some of these are summarized in this article. We will focus first on bacterial infection, and most notably the role of gut microbiota in regulaton of both obesity and inflammation. In particular, we focus on the role of inflammasomes and propose that understanding the role of Toll-like receptors and Nod-like receptors in the pathogenesis of inflammatory disorders with or without infection may provide novel targets for prevention and/or treatment of associated diseases. Secondly, chronic bacterial or viral infection and emerging links with metabolism will be reviewed. Finally, consideratons of biomarkers for metabolic syndrome, in particular lipocalin-2, and their link with infection will be discussed.
Keyword
MeSH Terms
Figure
Reference
-
1. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009. 120:1640–1645.2. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006. 444:860–867.3. Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977. 31:107–133.4. Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004. 101:15718–15723.5. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005. 102:11070–11075.6. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009. 457:480–484.7. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006. 444:1027–1031.8. Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006. 7:1250–1257.9. Fritz JH, Le Bourhis L, Magalhaes JG, Philpott DJ. Innate immune recognition at the epithelial barrier drives adaptive immunity: APCs take the back seat. Trends Immunol. 2008. 29:41–49.10. Kanczkowski W, Ziegler CG, Zacharowski K, Bornstein SR. Toll-like receptors in endocrine disease and diabetes. Neuroimmunomodulation. 2008. 15:54–60.11. Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009. 21:242–253.12. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006. 116:3015–3025.13. Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010. 328:228–231.14. Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009. 19:455–464.15. Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010. 327:296–300.16. Netea MG, van de Veerdonk FL, Kullberg BJ, Van der Meer JW, Joosten LA. The role of NLRs and TLRs in the activation of the inflammasome. Expert Opin Biol Ther. 2008. 8:1867–1872.17. Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009. 27:229–265.18. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010. 11:136–140.19. Maedler K, Dharmadhikari G, Schumann DM, Storling J. Interleukin-1 beta targeted therapy for type 2 diabetes. Expert Opin Biol Ther. 2009. 9:1177–1188.20. Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009. 32:1663–1668.21. Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007. 356:1517–1526.22. Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000. 85:140–146.23. Nabipour I, Vahdat K, Jafari SM, Pazoki R, Sanjdideh Z. The association of metabolic syndrome and Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, and herpes simplex virus type 1: the Persian Gulf Healthy Heart Study. Cardiovasc Diabetol. 2006. 5:25.24. El-Sadr WM, Mullin CM, Carr A, Gibert C, Rappoport C, Visnegarwala F, Grunfeld C, Raghavan SS. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005. 6:114–121.25. Diamond DL, Syder AJ, Jacobs JM, Sorensen CM, Walters KA, Proll SC, McDermott JE, Gritsenko MA, Zhang Q, Zhao R, Metz TO, Camp DG 2nd, Waters KM, Smith RD, Rice CM, Katze MG. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010. 6:e1000719.26. Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008. 47:2127–2133.27. Rasheed S, Yan JS, Lau A, Chan AS. HIV replication enhances production of free fatty acids, low density lipoproteins and many key proteins involved in lipid metabolism: a proteomics study. PLoS One. 2008. 3:e3003.28. Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: the role of cytokines. Ann N Y Acad Sci. 2006. 1084:89–117.29. Bezante GP, Briatore L, Rollando D, Maggi D, Setti M, Ghio M, Agosti S, Murdaca G, Balbi M, Barsotti A, Cordera R. Hypoadiponectinemia in lipodystrophic HIV individuals: a metabolic marker of subclinical cardiac damage. Nutr Metab Cardiovasc Dis. 2009. 19:277–282.30. Nystrom T. C-reactive protein: a marker or a player? Clin Sci (Lond). 2007. 113:79–81.31. Alpizar-Alpizar W, Laerum OD, Illemann M, Ramirez JA, Arias A, Malespin-Bendana W, Ramirez V, Lund LR, Borregaard N, Nielsen BS. Neutrophil gelatinase-associated lipocalin (NGAL/Lcn2) is upregulated in gastric mucosa infected with Helicobacter pylori. Virchows Arch. 2009. 455:225–233.32. Landro L, Damas JK, Flo TH, Heggelund L, Ueland T, Tjonnfjord GE, Espevik T, Aukrust P, Froland SS. Decreased serum lipocalin-2 levels in human immunodeficiency virus-infected patients: increase during highly active anti-retroviral therapy. Clin Exp Immunol. 2008. 152:57–63.33. Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006. 103:1834–1839.34. Halaas O, Steigedal M, Haug M, Awuh JA, Ryan L, Brech A, Sato S, Husebye H, Cangelosi GA, Akira S, Strong RK, Espevik T, Flo TH. Intracellular Mycobacterium avium intersect transferrin in the Rab11(+) recycling endocytic pathway and avoid lipocalin 2 trafficking to the lysosomal pathway. J Infect Dis. 2010. 201:783–792.35. Cowland JB, Muta T, Borregaard N. IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J Immunol. 2006. 176:5559–5566.36. Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005. 77:388–399.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Severity of Coronary Atherosclerosis; Influence of Metabolic Syndrome Risk Factor Clustering and hs-CRP
- Letter: Association between Exercise and Metabolic Syndrome in Koreans (J Obes Metab Syndr 2018;27:117-24)
- Mitochondrial Dysfunction and Insulin Resistance: The Contribution of Dioxin-Like Substances
- Effects of Functional Food Components in Reducing Obesity-induced Inflammation and Metabolic Diseases
- Mechanistic modelling for African swine fever transmission in the Republic of Korea