Immune Netw.
2013 Aug;13(4):123-132. 10.4110/in.2013.13.4.123.
Metformin Down-regulates TNF-alpha Secretion via Suppression of Scavenger Receptors in Macrophages
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul 139-742, Korea. kimkj@syu.ac.kr
- 2Department of Biology, Seoul Women's University, Seoul 139-774, Korea.
- KMID: 2150777
- DOI: http://doi.org/10.4110/in.2013.13.4.123
Abstract
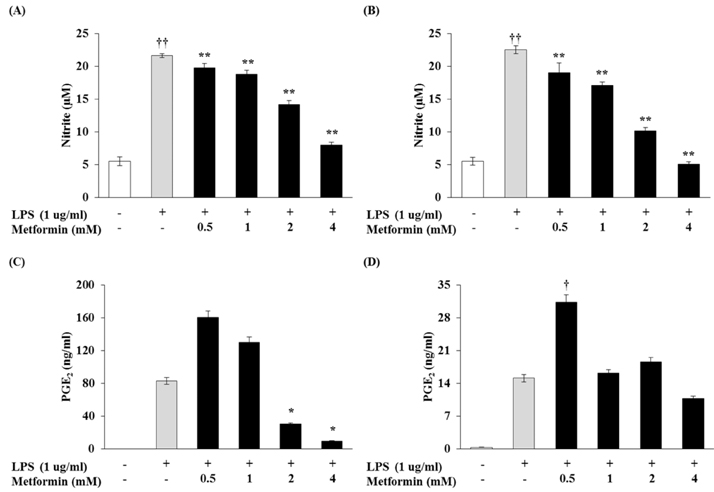
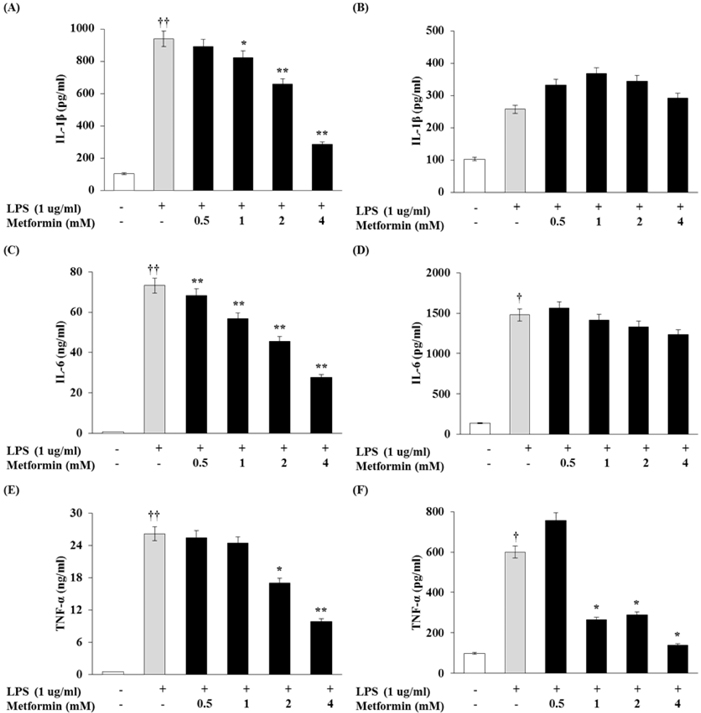
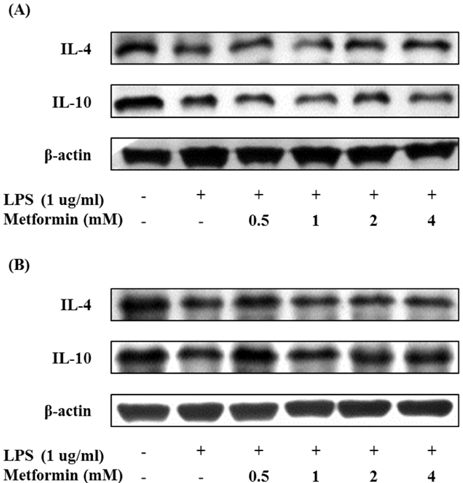
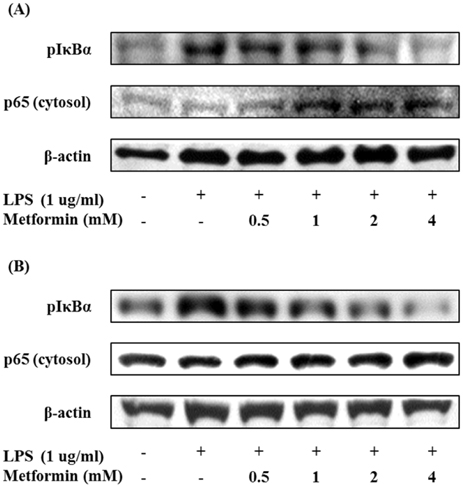
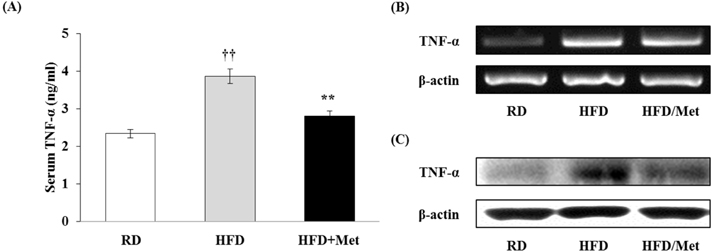
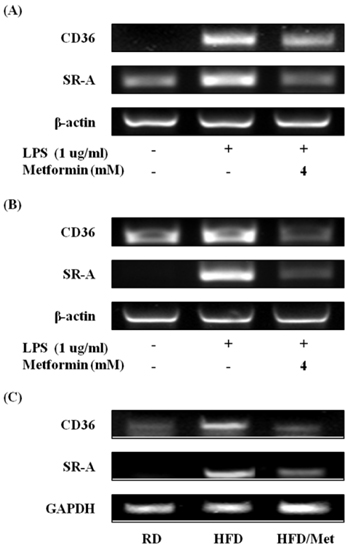
- Obesity is consistently increasing in prevalence and can trigger insulin resistance and type 2 diabetes. Many lines of evidence have shown that macrophages play a major role in inflammation associated with obesity. This study was conducted to determine metformin, a widely prescribed drug for type 2 diabetes, would regulate inflammation through down-regulation of scavenger receptors in macrophages from obesity-induced type 2 diabetes. RAW 264.7 cells and peritoneal macrophages were stimulated with LPS to induce inflammation, and C57BL/6N mice were fed a high-fat diet to generate obesity-induced type 2 diabetes mice. Metformin reduced the production of NO, PGE2 and pro-inflammatory cytokines (IL-1beta, IL-6 and TNF-alpha) through down-regulation of NF-kappaB translocation in macrophages in a dose-dependent manner. On the other hand, the protein expressions of anti-inflammatory cytokines, IL-4 and IL-10, were enhanced or maintained by metformin. Also, metformin suppressed secretion of TNF-alpha and reduced the protein and mRNA expression of TNF-alpha in obese mice as well as in macrophages. The expression of scavenger receptors, CD36 and SR-A, were attenuated by metformin in macrophages and obese mice. These results suggest that metformin may attenuate inflammatory responses by suppressing the production of TNF-alpha and the expressions of scavenger receptors.
MeSH Terms
-
Animals
Cytokines
Diet, High-Fat
Dinoprostone
Down-Regulation
Hand
Inflammation
Insulin Resistance
Interleukin-10
Interleukin-4
Interleukin-6
Macrophages
Macrophages, Peritoneal
Metformin
Mice
Mice, Obese
NF-kappa B
Obesity
Prevalence
Receptors, Scavenger
RNA, Messenger
Tumor Necrosis Factor-alpha
Cytokines
Dinoprostone
Interleukin-10
Interleukin-4
Interleukin-6
Metformin
NF-kappa B
RNA, Messenger
Receptors, Scavenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010; 303:235–241.
Article2. Bae NK, Kwon IS, Cho YC. Ten year change of body mass index in Korean: 1997-2007. Korean J Obes. 2009; 18:24–30.3. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003; 289:76–79.
Article4. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004; 89:2595–2600.
Article5. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87:4–14.
Article6. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–553.
Article7. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007; 132:2169–2180.
Article8. Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007; 21:1443–1455.
Article9. McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne). 2013; 4:52.
Article10. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010; 72:219–246.
Article11. Kim JY. Patho-/physiological roles of adipose tissue macrophages. BioWave. 2010; 12:1–12.12. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011; 11:738–749.
Article13. Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012; 18:363–374.
Article14. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117:175–184.
Article15. Patel PS, Buras ED, Balasubramanyam A. The role of the immune system in obesity and insulin resistance. J Obes. 2013; 616193.
Article16. Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979; 76:333–337.
Article17. Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011; 31:1506–1516.
Article18. Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012; 217:492–502.
Article19. Baranova IN, Kurlander R, Bocharov AV, Vishnyakova TG, Chen Z, Remaley AT, Csako G, Patterson AP, Eggerman TL. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J Immunol. 2008; 181:7147–7156.
Article20. Chen Y, Wermeling F, Sundqvist J, Jonsson AB, Tryggvason K, Pikkarainen T, Karlsson MC. A regulatory role for macrophage class A scavenger receptors in TLR4-mediated LPS responses. Eur J Immunol. 2010; 40:1451–1460.
Article21. Yu H, Ha T, Liu L, Wang X, Gao M, Kelley J, Kao R, Williams D, Li C. Scavenger receptor A (SR-A) is required for LPS-induced TLR4 mediated NF-κB activation in macrophages. Biochim Biophys Acta. 2012; 1823:1192–1198.
Article22. Cai L, Wang Z, Ji A, Meyer JM, van der Westhuyzen DR. Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS ONE. 2012; 7:e36785.
Article23. Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996; 334:574–579.
Article24. Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000; 49:2063–2069.
Article25. Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs. 2003; 63:1879–1894.26. Correia S, Carvalho C, Santos MS, Seiça R, Oliveira CR, Moreira PI. Mechanisms of action of metformin in type 2 diabetes and associated complications: an overview. Mini Rev Med Chem. 2008; 8:1343–1354.
Article27. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108:1167–1174.
Article28. Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002; 51:2074–2081.
Article29. Lee SK, Lee JO, Kim JH, Kim SJ, You GY, Moon JW, Jung JH, Park SH, Uhm KO, Park JM, Suh PG, Kim HS. Metformin sensitizes insulin signaling through AMPK-mediated PTEN down-regulation in preadipocyte 3T3-L1 cells. J Cell Biochem. 2011; 112:1259–1267.
Article30. Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009; 182:8005–8014.
Article31. Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol. 2011; 162:1498–1508.
Article32. Kalariya NM, Shoeb M, Ansari NH, Srivastava SK, Ramana KV. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2012; 53:3431–3440.
Article33. Yuan H, Li L, Zheng W, Wan J, Ge P, Li H, Zhang L. Antidiabetic drug metformin alleviates endotoxin-induced fulminant liver injury in mice. Int Immunopharmacol. 2012; 12:682–688.
Article34. Kim HK, Lee IK. Endoplasmic reticulum (ER) stress and vascular complication. J Korean Diabetes Assoc. 2006; 30:145–150.
Article35. Serhan CN, Levy B. Success of prostaglandin E2 in structure-function is a challenge for structure-based therapeutics. Proc Natl Acad Sci U S A. 2003; 100:8609–8611.
Article36. Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997; 100:2417–2423.
Article37. Sun L, Liu J, Cui D, Li J, Yu Y, Ma L, Hu L. Anti-inflammatory function of Withangulatin A by targeted inhibiting COX-2 expression via MAPK and NF-kappaB pathways. J Cell Biochem. 2010; 109:532–541.38. Vila-del Sol V, Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J Immunol. 2005; 174:2825–2833.
Article39. Dinarello CA. Proinflammatory cytokines. Chest. 2000; 118:503–508.
Article40. Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006; 103:2274–2279.
Article41. Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000; 117:1162–1172.
Article42. Ma X. TNF-alpha and IL-12: a balancing act in macrophage functioning. Microbes Infect. 2001; 3:121–129.43. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001; 104:487–501.44. Maini RN, Taylor PC. Anti-cytokine therapy for rheumatoid arthritis. Annu Rev Med. 2000; 51:207–229.
Article45. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003; 3:745–756.
Article46. Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, Balkwill FR, Hagemann T. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009; 119:3011–3023.
Article47. Ji ZZ, Dai Z, Xu YC. A new tumor necrosis factor (TNF)-α regulator, lipopolysaccharides-induced TNF-α factor, is associated with obesity and insulin resistance. Chin Med J (Engl). 2011; 124:177–182.48. Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994; 91:4854–4858.
Article49. Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D'Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002; 105:804–809.
Article50. Simons PJ, van den Pangaart PS, Aerts JM, Boon L. Pro-inflammatory delipidizing cytokines reduce adiponectin secretion from human adipocytes without affecting adiponectin oligomerization. J Endocrinol. 2007; 192:289–299.
Article51. Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007; 48:751–762.
Article52. Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008; 79:360–376.
Article53. Skoog T, Dichtl W, Boquist S, Skoglund-Andersson C, Karpe F, Tang R, Bond MG, de Faire U, Nilsson J, Eriksson P, Hamsten A. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur Heart J. 2002; 23:376–383.
Article54. Mei CL, Chen ZJ, Liao YH, Wang YF, Peng HY, Chen Y. Interleukin-10 inhibits the down-regulation of ATP binding cassette transporter A1 by tumour necrosis factor-alpha in THP-1 macrophage-derived foam cells. Cell Biol Int. 2007; 31:1456–1461.
Article55. Hashizume M, Mihara M. Atherogenic effects of TNF-α and IL-6 via up-regulation of scavenger receptors. Cytokine. 2012; 58:424–430.
Article56. Spagnoli LG, Bonanno E, Sangiorgi G, Mauriello A. Role of inflammation in atherosclerosis. J Nucl Med. 2007; 48:1800–1815.
Article57. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001; 107:7–11.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Scavenger Receptor Class A to E Involved in Various Cancers
- TNF-alpha and IL-12 Secretion in Macrophages in Response to Spores of Bacillus anthracis Sterne
- The Effects of Air-borne Particulate Matters on the Alveolar Macrophages for the TNF-alpha and IL-1beta Secretion
- An Immunohistochemical Study on the Macrophages in the Developing Corpora Lutea of the Rat
- Role of Clusterin and Tumor Necrosis Factor Receptors on the Apoptosis of Prostate Cancer Cells