Korean J Physiol Pharmacol.
2012 Oct;16(5):321-326. 10.4196/kjpp.2012.16.5.321.
Antiproliferative and Cytotoxic Effects of Resveratrol in Mitochondria-Mediated Apoptosis in Rat B103 Neuroblastoma Cells
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Institute of Natural Medicine, Hallym University, Chuncheon 200-702, Korea. s0huh@hallym.ac.kr
- KMID: 1493964
- DOI: http://doi.org/10.4196/kjpp.2012.16.5.321
Abstract
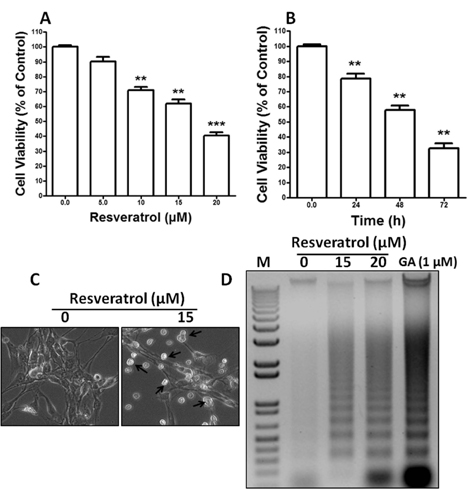
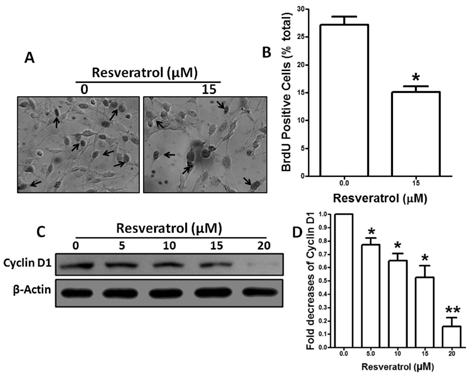
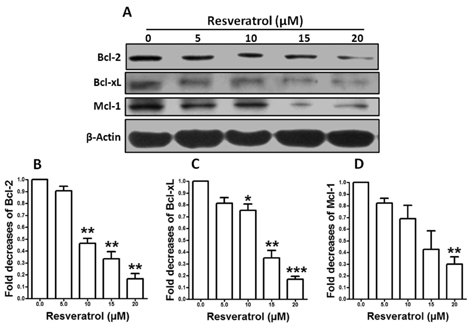
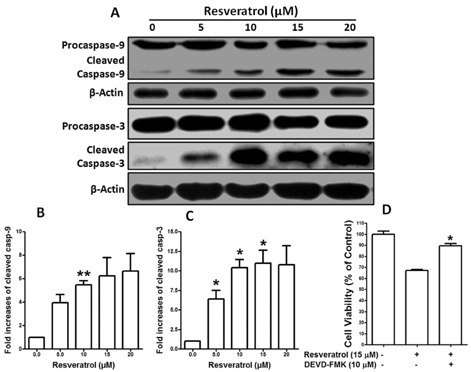
- Resveratrol, a natural compound, has been shown to possess anti-cancer, anti-aging, anti-inflammatory, anti-microbial, and neuroprotective activities. In this study, we examined the antiproliferative and cytotoxicity properties of resveratrol in Rat B103 neuroblastoma cells; although it's molecular mechanisms for the biological effects are not fully defined. Here, we examined the cellular cytotoxicity of resveratrol by cell viability assay, antiproliferation by BrdU assay, DNA fragmentation by DNA ladder assay, activation of caspases and Bcl-2 family proteins were detected by western blot analyses. The results of our investigation suggest that resveratrol increased cellular cytotoxicity of Rat B103 neuroblastoma cells in a dose-and time-dependent manner with IC50 of 17.86 microM at 48 h. On the other hand, incubation of neuroblastoma cells with resveratrol resulted in S-phase cell cycle arrests which dose-dependently and significantly reduced BrdU positive cells through the downregulation of cyclin D1 protein. In addition, resveratrol dose-dependently and significantly downregulated the expression of anti-apoptotic protein includes Bcl-2, Bcl-xL and Mcl-1 and also activates cleavage caspase-9 and-3 via the downregulation of procaspase-9 and -3 in a dose-dependent manner which indicates that involvement of intrinsic mitochondria-mediated apoptotic pathway. In conclusion, resveratrol increases cellular cytotoxicity and inhibits the proliferation of B103 neuroblastoma cells by inducing mitochondria-mediated intrinsic caspase dependent pathway which suggests this natural compound could be used as therapeutic purposes for neuroblastoma malignancies.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Resveratrol attenuates lipopolysaccharide-induced dysfunction of blood-brain barrier in endothelial cells via AMPK activation
Min Hu, Bo Liu
Korean J Physiol Pharmacol. 2016;20(4):325-332. doi: 10.4196/kjpp.2016.20.4.325.
Reference
-
1. Bénard J, Raguénez G, Kauffmann A, Valent A, Ripoche H, Joulin V, Job B, Danglot G, Cantais S, Robert T, Terrier-Lacombe MJ, Chassevent A, Koscielny S, Fischer M, Berthold F, Lipinski M, Tursz T, Dessen P, Lazar V, Valteau-Couanet D. MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: a molecular portrait of stage 4S. Mol Oncol. 2008. 2:261–271.2. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007. 369:2106–2120.3. Castel V, Grau E, Noguera R, Martínez F. Molecular biology of neuroblastoma. Clin Transl Oncol. 2007. 9:478–483.4. Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009. 53:115–128.5. Roccaro AM, Leleu X, Sacco A, Moreau AS, Hatjiharissi E, Jia X, Xu L, Ciccarelli B, Patterson CJ, Ngo HT, Russo D, Vacca A, Dammacco F, Anderson KC, Ghobrial IM, Treon SP. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenström's macroglobulinemia. Clin Cancer Res. 2008. 14:1849–1858.6. Pallàs M, Casadesús G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009. 6:70–81.7. Pallàs M, Verdaguer E, Tajes M, Gutierrez-Cuesta J, Camins A. Modulation of sirtuins: new targets for antiageing. Recent Pat CNS Drug Discov. 2008. 3:61–69.8. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997. 275:218–220.9. Pozo-Guisado E, Lorenzo-Benayas MJ, Fernández-Salguero PM. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: relevance in cell proliferation. Int J Cancer. 2004. 109:167–173.10. Komina O, Wesierska-Gadek J. Action of resveratrol alone or in combination with roscovitine, a CDK inhibitor, on cell cycle progression in human HL-60 leukemia cells. Biochem Pharmacol. 2008. 76:1554–1562.11. Roy P, Kalra N, Prasad S, George J, Shukla Y. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharm Res. 2009. 26:211–217.12. Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004. 24:2783–2840.13. Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997. 278:1073–1077.14. Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000. 130:2S Suppl. 467S–471S.15. Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000. 21:525–530.16. Clément MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998. 92:996–1002.17. Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clin Biochem. 1997. 30:91–113.18. Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004. 16:139–144.19. Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004. 23:2950–2966.20. Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999. 274:20049–20052.21. Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005. 115:2665–2672.22. Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999. 428:305–327.23. Carbó N, Costelli P, Baccino FM, López-Soriano FJ, Argilés JM. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophys Res Commun. 1999. 254:739–743.24. Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000. 256:50–57.25. Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999. 17:2941–2953.26. Bertoncello I, Bradley TR, Watt SM. An improved negative immunomagnetic selection strategy for the purification of primitive hemopoietic cells from normal bone marrow. Exp Hematol. 1991. 19:95–100.27. Buick RN, Till JE, McCulloch EA. Colony assay for proliferative blast cells circulating in myeloblastic leukaemia. Lancet. 1977. 1:862–863.28. Minden MD, Buick RN, McCulloch EA. Separation of blast cell and T-lymphocyte progenitors in the blood of patients with acute myeloblastic leukemia. Blood. 1979. 54:186–195.29. Datta R, Banach D, Kojima H, Talanian RV, Alnemri ES, Wong WW, Kufe DW. Activation of the CPP32 protease in apoptosis induced by 1-beta-D-arabinofuranosylcytosine and other DNAdamaging agents. Blood. 1996. 88:1936–1943.30. Ibrado AM, Huang Y, Fang G, Liu L, Bhalla K. Overexpression of Bcl-2 or Bcl-xL inhibits Ara-C-induced CPP32/Yama protease activity and apoptosis of human acute myelogenous leukemia HL-60 cells. Cancer Res. 1996. 56:4743–4748.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Flow Cytometric Analysis of the Effects of Resveratrol on the Survival of Human Tennon's Capsule Fibroblasts
- Apoptotic Effects of Melandryum firmum Root Extracts in Human SH-SY5Y Neuroblastoma Cells
- Sensitization of TNF alpha and Agonistic FAS/CD95 Antibody-Induced Apoptosis by INF gamma on Neuroblastoma Cells
- Neuroprotective effects of resveratrol on 6-hydroxydopamine-induced damage of SH-SY5Y cell line
- Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures