J Clin Neurol.
2010 Sep;6(3):127-137. 10.3988/jcn.2010.6.3.127.
Melatonin Potentiates the Neuroprotective Properties of Resveratrol Against Beta-Amyloid-Induced Neurodegeneration by Modulating AMP-Activated Protein Kinase Pathways
- Affiliations
-
- 1Department of Neurology, Center for Geriatric Neuroscience Research, Institute of Biomedical Science and Technology, School of Medicine, Konkuk University, Seoul, Korea. alzdoc@kuh.ac.kr
- 2Department of Neurology, Hanyang University College of Medicine, Seoul, Korea.
- 3Department of Pharmacology, Center for Geriatric Neuroscience Research, Institute of Biomedical Science and Technology, School of Medicine, Konkuk University, Seoul, Korea.
- KMID: 1462848
- DOI: http://doi.org/10.3988/jcn.2010.6.3.127
Abstract
- BACKGROUND AND PURPOSE
Recent studies have demonstrated that resveratrol (RSV) reduces the incidence of age-related macular degeneration, Alzheimer's disease (AD), and stroke, while melatonin (MEL) supplementation reduces the progression of the cognitive impairment in AD patients. The purpose of this investigation was to assess whether the co-administration of MEL and RSV exerts synergistic effects on their neuroprotective properties against beta-amyloid (Abeta)-induced neuronal death.
METHODS
The neuroprotective effects of co-treatment with MEL and RSV on Abeta1-42 -induced cell death, was measured by MTT reduction assay. Abeta1-42 caused an increase in intracellular levels of reactive oxygen species (ROS), as assessed by H2-DCF-DA dye, and a reduction of total glutathione (GSH) levels and mitochondrial membrane potential, as assessed using monochlorobimane and rhodamine 123 fluorescence, respectively. Western blotting was used to investigate the intracellular signaling mechanism involved in these synergic effects.
RESULTS
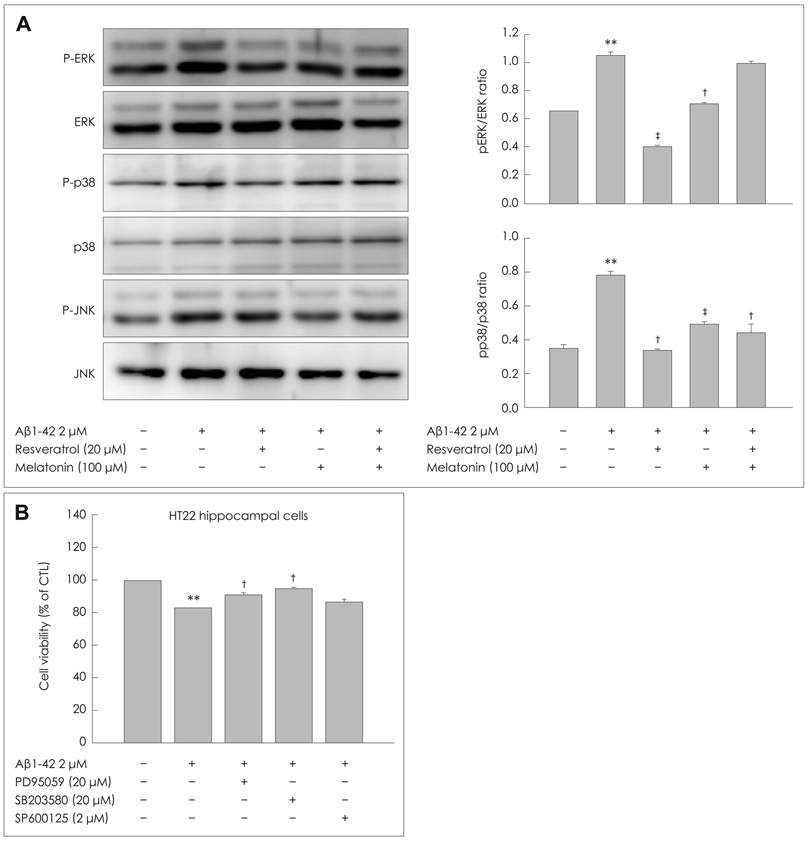
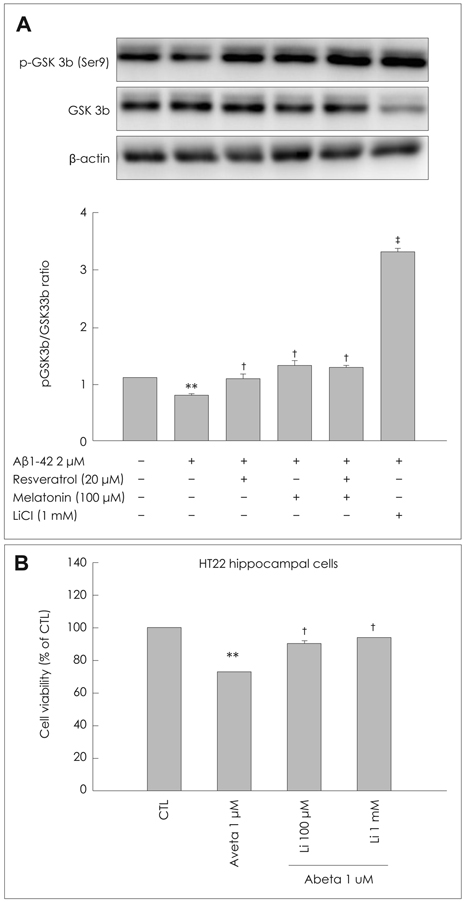
We treated a murine HT22 hippocampal cell line with MEL or RSV alone or with both simultaneously. MEL and RSV alone significantly attenuated ROS production, mitochondrial membrane-potential disruption and the neurotoxicity induced by Abeta1-42. They also restored the Abeta1-42-induced depletion of GSH, back to within its normal range and prevented the Abeta1-42-induced activation of glycogen synthase kinase 3beta (GSK3beta). However, co-treatment with MEL and RSV did not exert any significant synergistic effects on either the recovery of the Abeta1-42-induced depletion of GSH or on the inhibition of Abeta1-42-induced GSK3beta activation. Abeta1-42 treatment increased AMP-activated protein kinase (AMPK) activity, which is associated with subsequent neuronal death. We demonstrated that MEL and RSV treatment inhibited the phosphorylation of AMPK.
CONCLUSIONS
Together, our results suggest that co-administration of MEL and RSV acts as an ef-fective treatment for AD by attenuating Abeta1-42-induced oxidative stress and the AMPK-dependent pathway.
Keyword
MeSH Terms
-
Alzheimer Disease
AMP-Activated Protein Kinases
Blotting, Western
Cell Death
Cell Line
Fluorescence
Glutathione
Glycogen Synthase Kinase 3
Glycogen Synthase Kinases
Humans
Incidence
Macular Degeneration
Melatonin
Membrane Potential, Mitochondrial
Neurons
Neuroprotective Agents
Oxidative Stress
Phosphorylation
Pyrazoles
Reactive Oxygen Species
Reference Values
Rhodamine 123
Stilbenes
Stroke
AMP-Activated Protein Kinases
Glutathione
Glycogen Synthase Kinase 3
Glycogen Synthase Kinases
Melatonin
Neuroprotective Agents
Pyrazoles
Reactive Oxygen Species
Rhodamine 123
Stilbenes
Figure
Reference
-
1. Kelley BJ, Petersen RC. Alzheimer's disease and mild cognitive impairment. Neurol Clin. 2007. 25:577–609.
Article2. Selkoe DJ. The molecular pathology of Alzhiemer's disease. Neuron. 1991. 6:487–498.3. Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004. 89:1313–1317.
Article4. Williams KW, Coppari R, Elmquist JK. "AMPing up" our understanding of the hypothalamic control of energy balance. J Clin Invest. 2007. 117:2089–2092.
Article5. Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991. 12:151–180.
Article6. Witt-Enderby PA, Radio NM, Doctor JS, Davis VL. Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J Pineal Res. 2006. 41:297–305.
Article7. Reiter RJ, Tan DX, Qi W, Manchester LC, Karbownik M, Calvo JR. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol Signals Recept. 2000. 9:160–171.
Article8. Antolín I, Mayo JC, Sainz RM, del Brío Mde L, Herrera F, Martín V, et al. Protective effect of melatonin in a chronic experimental model of Parkinson's disease. Brain Res. 2002. 943:163–173.
Article9. Shen YX, Xu SY, Wei W, Wang XL, Wang H, Sun X. Melatonin blocks rat hippocampal neuronal apoptosis induced by amyloid beta-peptide 25-35. J Pineal Res. 2002. 32:163–167.
Article10. Costa EJ, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995. 19:123–126.
Article11. Pierpaoli W, Regelson W. Pineal control of aging: effect of melatonin and pineal grafting on aging mice. Proc Natl Acad Sci U S A. 1994. 91:787–791.
Article12. Reiter RJ, Pablos MI, Agapito TT, Guerrero JM. Melatonin in the context of the free radical theory of aging. Ann N Y Acad Sci. 1996. 786:362–378.
Article13. Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J, et al. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995. 18:1–11.
Article14. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006. 5:493–506.
Article15. Zhuang H, Kim YS, Koehler RC, Doré S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann N Y Acad Sci. 2003. 993:276–286.
Article16. Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, et al. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006. 78:2564–2570.
Article17. Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007. 73:550–560.
Article18. Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005. 280:37377–37382.19. Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006. 16:296–300.
Article20. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006. 5:493–506.
Article21. Han SH. Potential role of sirtuin as a therapeutic target for neurodegenerative diseases. J Clin Neurol. 2009. 5:120–125.
Article22. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006. 444:337–342.
Article23. Mook-Jung I, Joo I, Sohn S, Kwon HJ, Huh K, Jung MW. Estrogen blocks neurotoxic effects of beta-amyloid (1-42) and induces neurite extension on B103 cells. Neurosci Lett. 1997. 235:101–104.
Article24. LeBel CP, Ali SF, McKee M, Bondy SC. Organometal-induced increases in oxygen reactive species: the potential of 2',7'-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol. 1990. 104:17–24.
Article25. Martín M, Macías M, León J, Escames G, Khaldy H, Acuña-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol. 2002. 34:348–357.
Article26. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Dau-ssin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006. 127:1109–1122.
Article27. BALANCE investigators and collaborators. Geddes JR, Goodwin GM, Rendell J, Azorin JM, Cipriani A, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010. 375:385–395.
Article28. Tajes M, Gutierrez-Cuesta J, Ortuño-Sahagun D, Camins A, Pallàs M. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J Pineal Res. 2009. 47:228–237.
Article29. Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK-1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006. 281:20315–20325.
Article30. Lee EO, Park HJ, Kang JL, Kim HS, Chong YH. Resveratrol reduces glutamate-mediated monocyte chemotactic protein-1 expression via inhibition of extracellular signal-regulated kinase 1/2 pathway in rat hippocampal slice cultures. J Neurochem. 2010. 112:1477–1487.
Article31. Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010. 285:9100–9133.32. Ha E, Yim SV, Chung JH, Yoon KS, Kang I, Cho YH, et al. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J Pineal Res. 2006. 41:67–72.
Article33. Kim SD, Yang SI, Kim HC, Shin CY, Ko KH. Inhibition of GSK-3beta mediates expression of MMP-9 through ERK1/2 activation and tr-anslocation of NF-kappaB in rat primary astrocyte. Brain Res. 2007. 1186:12–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Resveratrol-induced Relaxation of Cholecystokinin Octapeptide- or KCl-induced Tension in Male Guinea Pig Gallbladder Strips Is Mediated Through L-type Ca2+ Channels
- Protein Kinase C-mediated Neuroprotective Action of (-)-epigallocatechin-3-gallate against Abeta1-42-induced Apoptotic Cell Death in SH-SY5Y Neuroblastoma Cells
- Letter to the Editor: 17Beta-estradiol Stimulates Glucose Uptake Through Estrogen Receptor and AMP-activated Protein Kinase Activation in C2C12 Myotubes (Korean J Obes 2016;25:190-6)
- Resveratrol attenuates lipopolysaccharide-induced dysfunction of blood-brain barrier in endothelial cells via AMPK activation
- Effects of AMP-activated Protein Kinase Activating Compounds and Its Mechanism