Korean J Physiol Pharmacol.
2020 Mar;24(2):137-147. 10.4196/kjpp.2020.24.2.137.
Lovastatin derivative dehydrolovastatin ameliorates ulcerative colitis in mice by suppressing NF-κB and inflammatory cytokine expression
- Affiliations
-
- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu City 610072, Sichuan Province, P.R. China. chenqiu1005@cdutcm.edu.cn
- 2Chongqing Medical and Pharmaceutical College, Chongqing Engineering Research Center of Pharmaceutical Sciences, Chongqing City 401331, P.R. China. dengqinghua2000@163.com
- 3People's Hospital of Shizhu County, Chongqing City 409100, P.R. China.
- KMID: 2471035
- DOI: http://doi.org/10.4196/kjpp.2020.24.2.137
Abstract
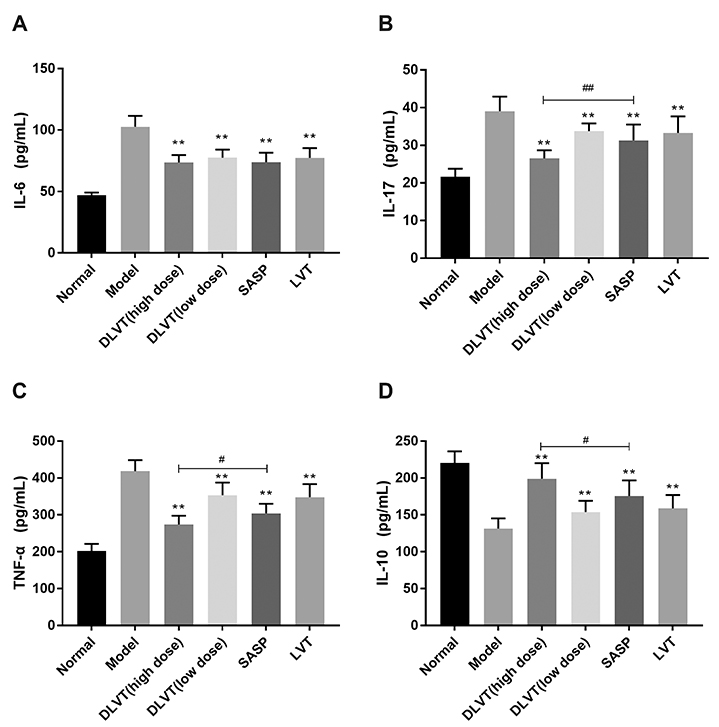
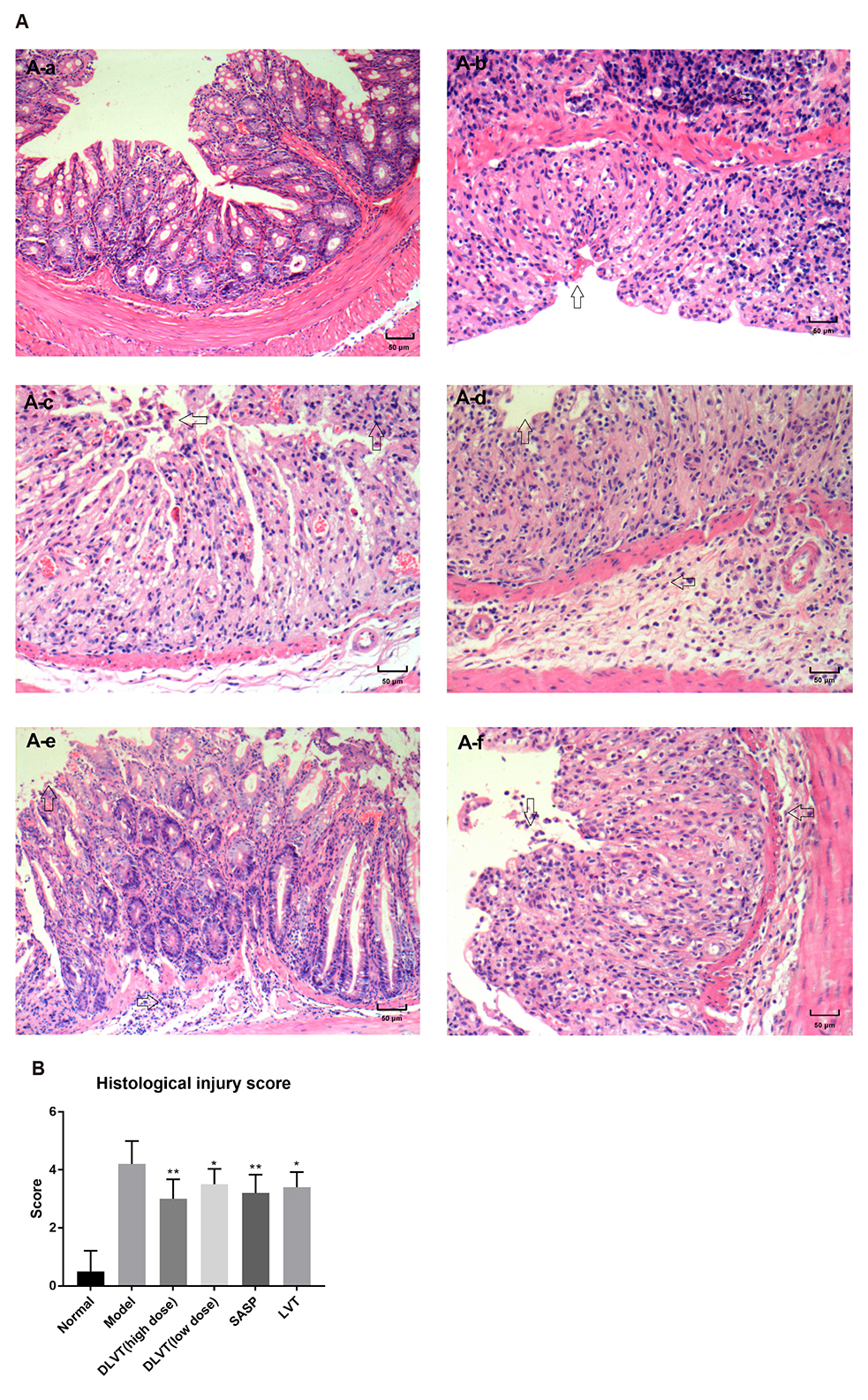
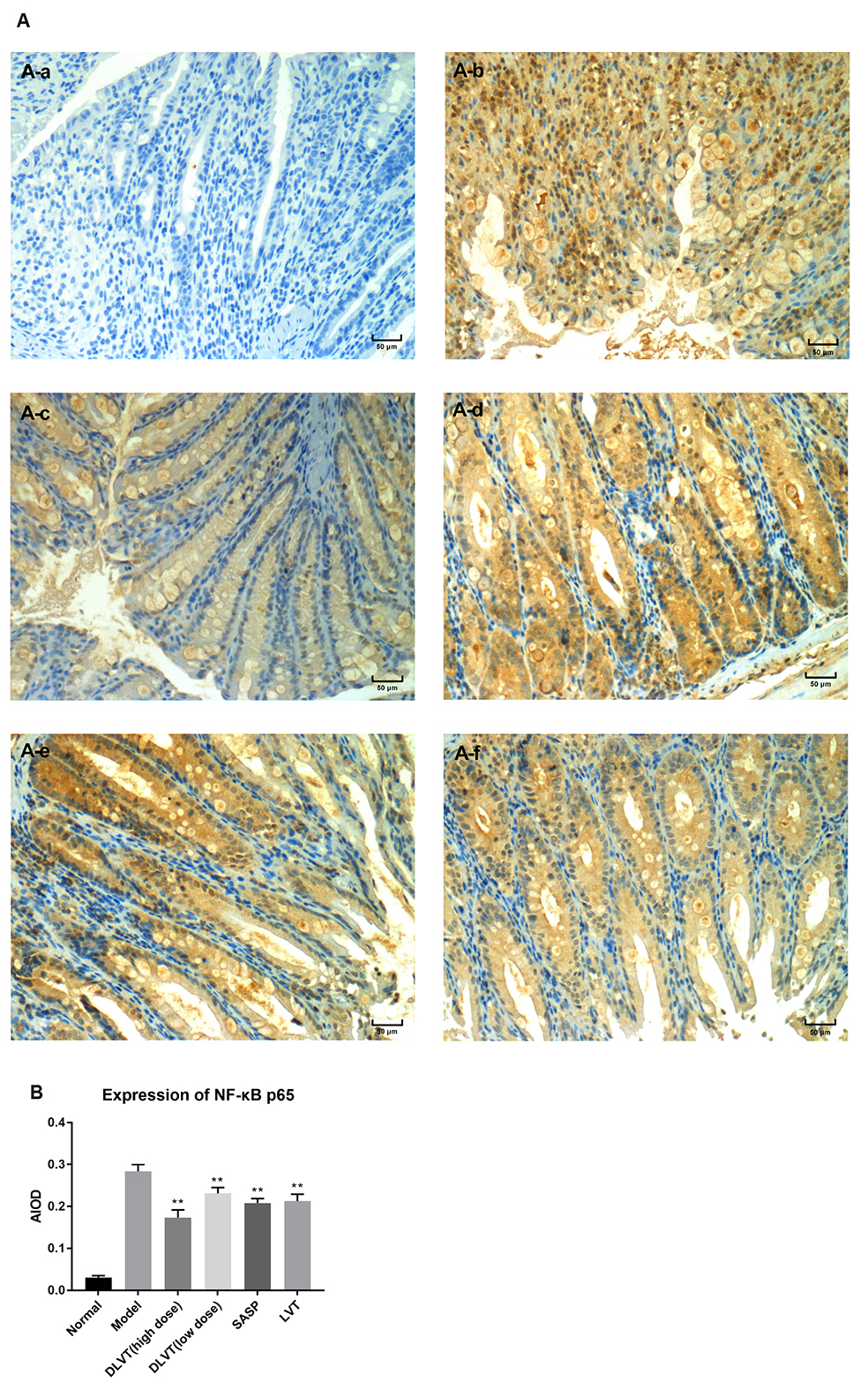
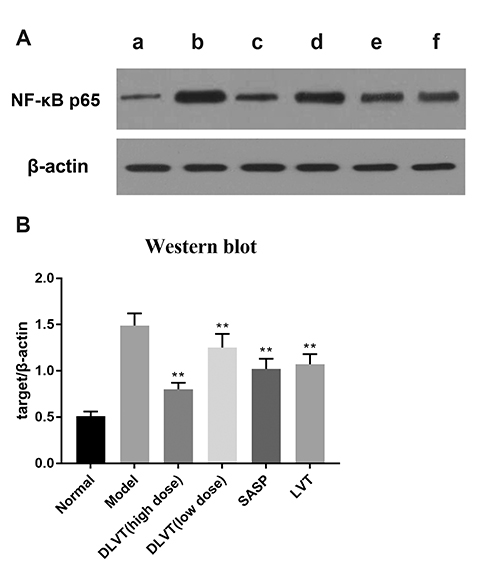
- Ulcerative colitis (UC) is associated with intestinal immune imbalance and inflammatory response. Because dehydrolovastatin (DLVT), a derivative of lovastatin, has been recently shown to inhibit inflammation and relieve immune arthritis induced by chemical stimuli, we studied its effect and possible mechanism on UC induced by dextran sulfate sodium. The BALB/c mice were classified into six groups: normal control group, model group, DLVT high dose group, DLVT low dose group, salazosulfapyridine (SASP) group and lovastatin (LVT) group. The disease activity indices of UC and pathological changes were investigated. The myeloperoxidase (MPO) activity in colon tissue and inflammatory factors such as IL-6, IL-10, IL-17, and TNF-α in the serum were analyzed by ELISA, while the expression of NF-κB p65 protein in colon tissue was detected by immunohistochemistry and western blot. DLVT relieved the disease activity indices and pathological damage of the UC mice. Furthermore, DLVT significantly decreased MPO activity and improved the imbalance of inflammatory cytokines through inhibiting the expression of NF-κB p65. Meanwhile, the positive drug of SASP has a similar effect to DLVT, but the effect of DLVT in both decreasing IL-17, TNF-α, and increasing IL-10 was significantly stronger than that of SASP. These results suggest that DLVT may ameliorates the symptoms of UC.
Keyword
MeSH Terms
-
Animals
Arthritis
Blotting, Western
Colitis, Ulcerative*
Colon
Cytokines
Dextran Sulfate
Enzyme-Linked Immunosorbent Assay
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Immunohistochemistry
Inflammation
Interleukin-10
Interleukin-17
Interleukin-6
Lovastatin*
Mice*
Peroxidase
Sulfasalazine
Ulcer*
Cytokines
Dextran Sulfate
Interleukin-10
Interleukin-17
Interleukin-6
Lovastatin
Peroxidase
Sulfasalazine
Figure
Reference
-
1. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011; 365:1713–1725.
Article2. Amati L, Caradonna L, Leandro G, Magrone T, Minenna M, Faleo G, Pellegrino NM, Jirillo E, Caccavo D. Immune abnormalities and endotoxemia in patients with ulcerative colitis and in their first degree relatives: attempts at neutralizing endotoxin-mediated effects. Curr Pharm Des. 2003; 9:1937–1945.3. Mitsuyama K, Toyonaga A, Sata M. Intestinal microflora as a therapeutic target in inflammatory bowel disease. J Gastroenterol. 2002; 37 Suppl 14:73–77.
Article4. Parlato M, Charbit-Henrion F, Hayes P, Tiberti A, Aloi M, Cucchiara S, Bègue B, Bras M, Pouliet A, Rakotobe S, Ruemmele F, Knaus UG, Cerf-Bensussan N. First identification of biallelic inherited DUOX2 inactivating mutations as a cause of very early onset inflammatory bowel disease. Gastroenterology. 2017; 153:609–611.e3.
Article5. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.
Article6. Low END, Mokhtar NM, Wong Z, Raja Ali RA. Colonic mucosal transcriptomic changes in patients with long-duration ulcerative colitis revealed colitis-associated cancer pathways. J Crohns Colitis. 2019; 13:755–763.
Article7. Matsuoka K, Kobayashi T, Ueno F, Matsui T, Hirai F, Inoue N, Kato J, Kobayashi K, Kobayashi K, Koganei K, Kunisaki R, Motoya S, Nagahori M, Nakase H, Omata F, Saruta M, Watanabe T, Tanaka T, Kanai T, Noguchi Y, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article8. Bernstein CN, Fried M, Krabshuis JH, Cohen H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG, LeMair AW, Malfertheiner , Ouyang Q, Rey JF, Sood A, Steinwurz F, Thomsen OO, Thomson A, Watermeyer G. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010; 16:112–124.
Article9. Piechota-Polanczyk A, Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 2014; 387:605–620.
Article10. Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014; 20:64–77.
Article11. Messaris E, Dassopoulos T. Concepts in inflammatory bowel disease management. In : Yeo CJ, editor. Shackelford's surgery of the alimentary tract. Philadelphia: Elsevier;2019. p. 1888–1918.12. Seidelin JB, Coskun M, Nielsen OH. Mucosal healing in ulcerative colitis: pathophysiology and pharmacology. Adv Clin Chem. 2013; 59:101–123.13. Pugliese D, Felice C, Papa A, Gasbarrini A, Rapaccini GL, Guidi L, Armuzzi A. Anti TNF-α therapy for ulcerative colitis: current status and prospects for the future. Expert Rev Clin Immunol. 2017; 13:223–233.
Article14. Ungaro R, Chang HL, Côté-Daigneault J, Mehandru S, Atreja A, Colombel JF. Statins associated with decreased risk of new onset inflammatory bowel disease. Am J Gastroenterol. 2016; 111:1416–1423.
Article15. Khalili H. Editorial: statins for inflammatory bowel disease: expanding the scope of prevention. Am J Gastroenterol. 2016; 111:1424–1426.
Article16. Côté-Daigneault J, Mehandru S, Ungaro R, Atreja A, Colombel JF. Potential immunomodulatory effects of statins in inflammatory bowel disease. Inflamm Bowel Dis. 2016; 22:724–732.
Article17. Li Y, Müller AL, Ngo MA, Sran K, Bellan D, Arora RC, Kirshenbaum LA, Freed DH. Statins impair survival of primary human mesenchymal progenitor cells via mevalonate depletion, NF-κB signaling, and Bnip3. J Cardiovasc Transl Res. 2015; 8:96–105.
Article18. Choi HW, Shin PG, Lee JH, Choi WS, Kang MJ, Kong WS, Oh MJ, Seo YB, Kim GD. Anti-inflammatory effect of lovastatin is mediated via the modulation of NF-κB and inhibition of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264.7 macrophages. Int J Mol Med. 2018; 41:1103–1109.
Article19. Deng Q, Zhouq Q, Chen Y, Jin L, He Q. Relationship between lipidemia-modulating and anti-inflammatory effects of dehydrolovastatin. Chin J N Drugs. 2011; 20:162–166.20. Deng Q, Yang Y, Zhou Q, Liu X, Gu Q. Experimental stdudy of dehydrolovastatin on adjuvant arthritis. Chongging Med J. 2015; 27:2320–2323.21. Pandurangan AK, Ismail S, Saadatdoust Z, Esa NM. Allicin alleviates dextran sodium sulfate- (DSS-) induced ulcerative colitis in BALB/c mice. Oxid Med Cell Longev. 2015; 2015:605208.
Article22. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993; 69:238–249.23. Togre N, Bhoj P, Goswami K, Tarnekar A, Patil M, Shende M. Human filarial proteins attenuate chronic colitis in an experimental mouse model. Parasite Immunol. 2018; 40:e12511.
Article24. Zhang D, Ren YB, Wu HG, Yang YT, Wu LJ, Zhang J, Shi Z, Ma XP. Effect of different doses of herbal cake-partitioned moxibustion on histopathological changes of colon tissue in ulcerative colitis rats. Zhen Ci Yan Jiu. 2018; 43:68–74.25. Agouridis AP, Elisaf MS, Nair DR, Mikhailidis DP. All for statins and statins for all; an update. Curr Pharm Des. 2016; 22:18–27.26. Moeinian M, Abdolghaffari AH, Nikfar S, Momtaz S, Abdollahi M. Effects of alpha lipoic acid and its derivative "andrographolid-lipoic acid-1" on ulcerative colitis: a systematic review with meta-analysis of animal studies. J Cell Biochem. 2019; 120:4766–4782.
Article27. Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008; 14:4280–4288.28. Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013; 36:379–386.
Article29. Qian J, Zhao W, Miao X, Li L, Zhang D. Sam68 modulates apoptosis of intestinal epithelial cells via mediating NF-κB activation in ulcerative colitis. Mol Immunol. 2016; 75:48–59.
Article30. Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J Gastroenterol. 2010; 16:4145–4151.31. Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T, Yoshikawa T, Kataoka K, Mazda O. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008; 377:12–16.
Article32. Zhang D, Wei C, Yao J, Cai X, Wang L. Interleukin-10 gene-carrying bifidobacteria ameliorate murine ulcerative colitis by regulating regulatory T cell/T helper 17 cell pathway. Exp Biol Med (Maywood). 2015; 240:1622–1629.
Article33. Masoodi I, Kochhar R, Dutta U, Vaishnavi C, Prasad KK, Vaiphei K, Hussain S, Singh K. Evaluation of fecal myeloperoxidase as a biomarker of disease activity and severity in ulcerative colitis. Dig Dis Sci. 2012; 57:1336–1340.
Article34. Garrity-Park M, Loftus EV Jr, Sandborn WJ, Smyrk TC. Myeloperoxidase immunohistochemistry as a measure of disease activity in ulcerative colitis: association with ulcerative colitis-colorectal cancer, tumor necrosis factor polymorphism and RUNX3 methylation. Inflamm Bowel Dis. 2012; 18:275–283.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Transcription Factor Decoy for NF-κB on the TNF-α Induced Cytokine and ICAM-1 Expression in Cultured HaCaT cells
- Pro-inflammatory cytokine expression through NF-kappaB/IkappaB pathway in lung epithelial cells
- Up-regulation of IGF Binding Protein-3 Inhibits Colonic Inflammatory Response
- The Preventive and Curative Effect of Cyanidin-3β-D-Glycoside and Its Metabolite Protocatechuic Acid Against TNBS-induced Colitis in Mice
- Upregulation of FcγRIIB by resveratrol via NF-κB activation reduces B-cell numbers and ameliorates lupus