Diabetes Metab J.
2019 Dec;43(6):854-866. 10.4093/dmj.2018.0179.
Metformin Ameliorates Lipotoxic β-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action
- Affiliations
-
- 1Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, and College of Medicine or College of Pharmacy, Seoul National University, Seoul, Korea. kspark@snu.ac.kr
- 2Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 3Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea.
- 4Division of Endocrinology, Diabetes and Metabolism, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
- 5Division of Nephrology, Department of Internal Medicine, Konyang University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2466506
- DOI: http://doi.org/10.4093/dmj.2018.0179
Abstract
- BACKGROUND
Chronic exposure to elevated levels of free fatty acids contributes to pancreatic β-cell dysfunction. Although it is well known that metformin induces cellular energy depletion and a concomitant activation of AMP-activated protein kinase (AMPK) through inhibition of the respiratory chain, previous studies have shown inconsistent results with regard to the action of metformin on pancreatic β-cells. We therefore examined the effects of metformin on pancreatic β-cells under lipotoxic stress.
METHODS
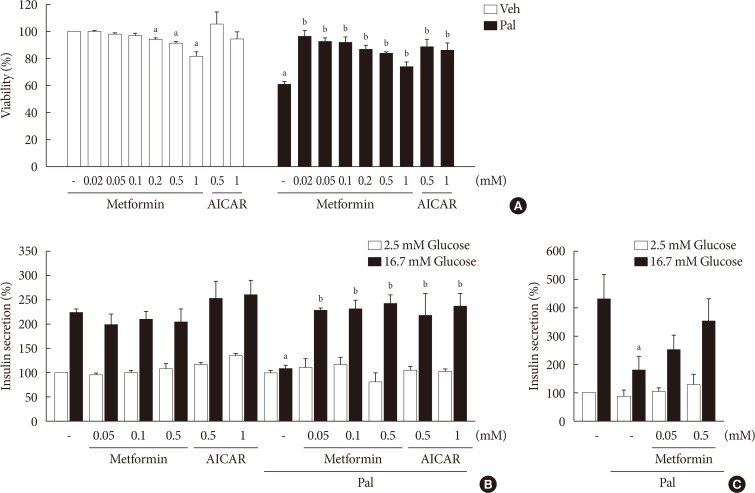
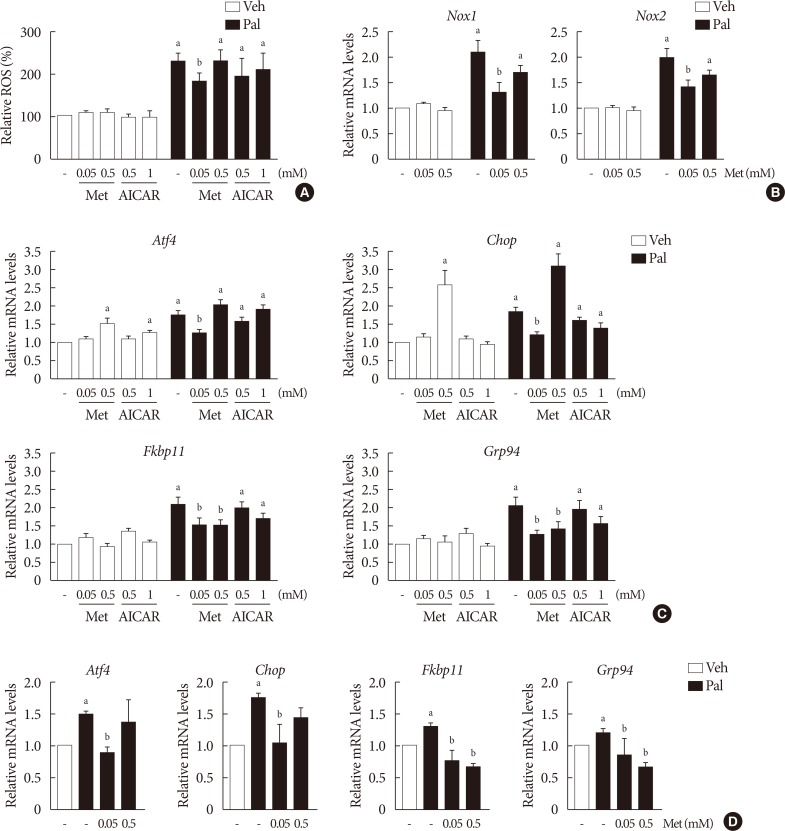
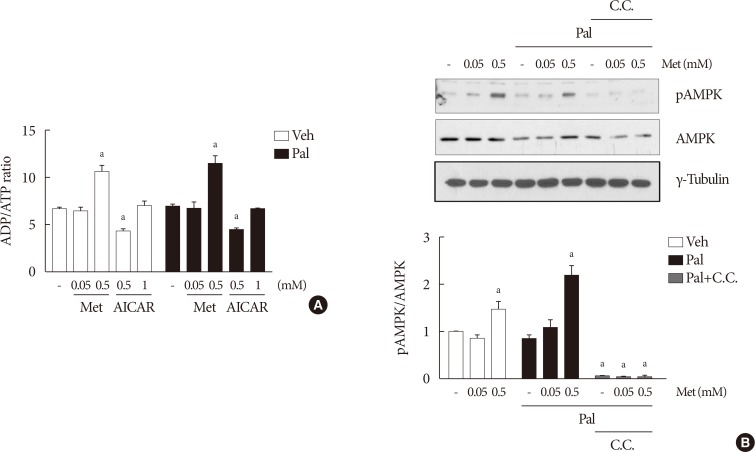
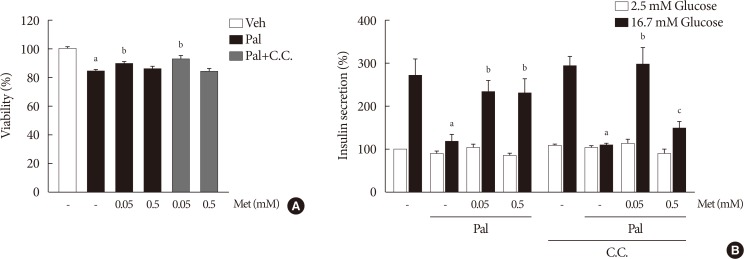
NIT-1 cells and mouse islets were exposed to palmitate and treated with 0.05 and 0.5 mM metformin. Cell viability, glucose-stimulated insulin secretion, cellular adenosine triphosphate, reactive oxygen species (ROS) levels and Rho kinase (ROCK) activities were measured. The phosphorylation of AMPK was evaluated by Western blot analysis and mRNA levels of endoplasmic reticulum (ER) stress markers and NADPH oxidase (NOX) were measured by real-time quantitative polymerase chain reaction analysis.
RESULTS
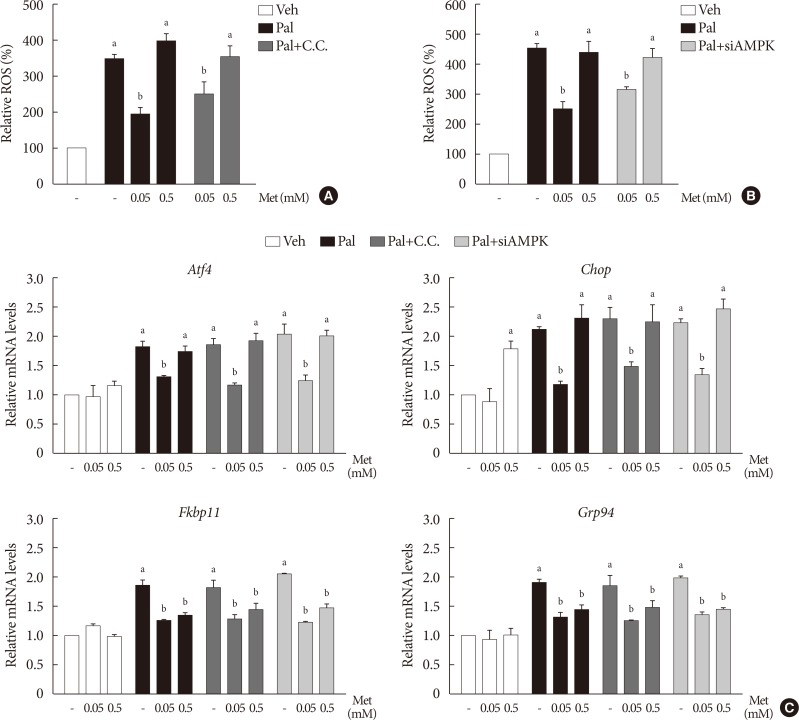
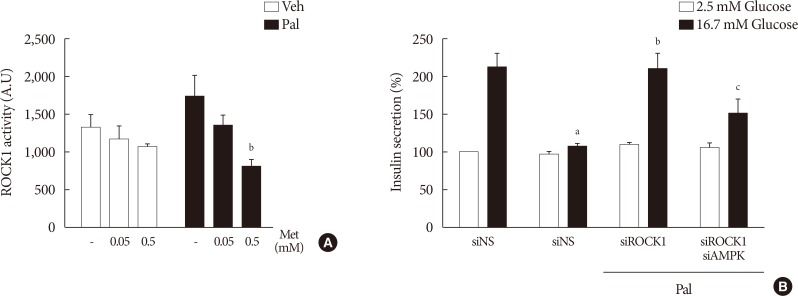
We found that metformin has protective effects on palmitate-induced β-cell dysfunction. Metformin at a concentration of 0.05 mM inhibits NOX and suppresses the palmitate-induced elevation of ER stress markers and ROS levels in a AMPK-independent manner, whereas 0.5 mM metformin inhibits ROCK activity and activates AMPK.
CONCLUSION
This study suggests that the action of metformin on β-cell lipotoxicity was implemented by different molecular pathways depending on its concentration. Metformin at a usual therapeutic dose is supposed to alleviate lipotoxic β-cell dysfunction through inhibition of oxidative stress and ER stress.
Keyword
MeSH Terms
-
Adenosine Triphosphate
AMP-Activated Protein Kinases
Animals
Blotting, Western
Cell Survival
Electron Transport
Endoplasmic Reticulum
Endoplasmic Reticulum Stress
Fatty Acids, Nonesterified
Insulin
Insulin-Secreting Cells
Metformin*
Mice
NADPH Oxidase
Oxidative Stress
Phosphorylation
Polymerase Chain Reaction
Reactive Oxygen Species
rho-Associated Kinases
RNA, Messenger
AMP-Activated Protein Kinases
Adenosine Triphosphate
Fatty Acids, Nonesterified
Insulin
Metformin
NADPH Oxidase
RNA, Messenger
Reactive Oxygen Species
rho-Associated Kinases
Figure
Reference
-
1. Elks ML. Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology. 1993; 133:208–214. PMID: 8319569.
Article2. El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003; 144:4154–4163. PMID: 12933690.3. Cnop M, Igoillo-Esteve M, Cunha DA, Ladriere L, Eizirik DL. An update on lipotoxic endoplasmic reticulum stress in pancreatic beta-cells. Biochem Soc Trans. 2008; 36:909–915. PMID: 18793160.4. Koshkin V, Wang X, Scherer PE, Chan CB, Wheeler MB. Mitochondrial functional state in clonal pancreatic beta-cells exposed to free fatty acids. J Biol Chem. 2003; 278:19709–19715. PMID: 12642585.5. Boni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009; 150:5218–5229. PMID: 19819943.6. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108:1167–1174. PMID: 11602624.
Article7. Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005; 310:1642–1646. PMID: 16308421.
Article8. Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O'Neill HM, Ford RJ, Palanivel R, O'Brien M, Hardie DG, Macaulay SL, Schertzer JD, Dyck JR, van Denderen BJ, Kemp BE, Steinberg GR. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013; 19:1649–1654. PMID: 24185692.
Article9. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000; 348 Pt 3:607–614. PMID: 10839993.
Article10. Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010; 120:2355–2369. PMID: 20577053.
Article11. Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014; 510:542–546. PMID: 24847880.
Article12. Patane G, Piro S, Rabuazzo AM, Anello M, Vigneri R, Purrello F. Metformin restores insulin secretion altered by chronic exposure to free fatty acids or high glucose: a direct metformin effect on pancreatic beta-cells. Diabetes. 2000; 49:735–740. PMID: 10905481.13. Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patanè G, Boggi U, Mosca F, Piro S, Del Prato S, Marchetti P. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes. 2002; 51 Suppl 1:S134–S137. PMID: 11815472.
Article14. Kefas BA, Cai Y, Kerckhofs K, Ling Z, Martens G, Heimberg H, Pipeleers D, Van de Casteele M. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem Pharmacol. 2004; 68:409–416. PMID: 15242807.15. Jiang Y, Huang W, Wang J, Xu Z, He J, Lin X, Zhou Z, Zhang J. Metformin plays a dual role in MIN6 pancreatic β-cell function through AMPK-dependent autophagy. Int J Biol Sci. 2014; 10:268–277. PMID: 24644425.16. Sum CF, Webster JM, Johnson AB, Catalano C, Cooper BG, Taylor R. The effect of intravenous metformin on glucose metabolism during hyperglycaemia in type 2 diabetes. Diabet Med. 1992; 9:61–65. PMID: 1551312.
Article17. Zmuda EJ, Powell CA, Hai T. A method for murine islet isolation and subcapsular kidney transplantation. J Vis Exp. 2011; (50):2096. PMID: 21525838.
Article18. Huang H, Lee SH, Ye C, Lima IS, Oh BC, Lowell BB, Zabolotny JM, Kim YB. ROCK1 in AgRP neurons regulates energy expenditure and locomotor activity in male mice. Endocrinology. 2013; 154:3660–3670. PMID: 23885017.
Article19. Akerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, King C, Biden TJ, Laybutt DR. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes. 2008; 57:3034–3044. PMID: 18591394.20. Simon-Szabo L, Kokas M, Mandl J, Keri G, Csala M. Metformin attenuates palmitate-induced endoplasmic reticulum stress, serine phosphorylation of IRS-1 and apoptosis in rat insulinoma cells. PLoS One. 2014; 9:e97868. PMID: 24896641.
Article21. Jung TW, Lee MW, Lee YJ, Kim SM. Metformin prevents endoplasmic reticulum stress-induced apoptosis through AMPK-PI3K-c-Jun NH2 pathway. Biochem Biophys Res Commun. 2012; 417:147–152. PMID: 22138650.
Article22. Quentin T, Steinmetz M, Poppe A, Thoms S. Metformin differentially activates ER stress signaling pathways without inducing apoptosis. Dis Model Mech. 2012; 5:259–269. PMID: 22107872.
Article23. Silva JM, Wong A, Carelli V, Cortopassi GA. Inhibition of mitochondrial function induces an integrated stress response in oligodendroglia. Neurobiol Dis. 2009; 34:357–365. PMID: 19233273.
Article24. Kim KH, Jeong YT, Kim SH, Jung HS, Park KS, Lee HY, Lee MS. Metformin-induced inhibition of the mitochondrial respiratory chain increases FGF21 expression via ATF4 activation. Biochem Biophys Res Commun. 2013; 440:76–81. PMID: 24041694.
Article25. Yuan H, Zhang X, Huang X, Lu Y, Tang W, Man Y, Wang S, Xi J, Li J. NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of β-cells via JNK, p38 MAPK and p53 pathways. PLoS One. 2010; 5:e15726. PMID: 21209957.
Article26. Tang C, Koulajian K, Schuiki I, Zhang L, Desai T, Ivovic A, Wang P, Robson-Doucette C, Wheeler MB, Minassian B, Volchuk A, Giacca A. Glucose-induced beta cell dysfunction in vivo in rats: link between oxidative stress and endoplasmic reticulum stress. Diabetologia. 2012; 55:1366–1379. PMID: 22396011.
Article27. Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008; 105:18525–18530. PMID: 19011102.
Article28. Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008; 118:3378–3389. PMID: 18776938.29. Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009; 10:13–26. PMID: 19583950.30. Egnatchik RA, Leamy AK, Noguchi Y, Shiota M, Young JD. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metabolism. 2014; 63:283–295. PMID: 24286856.
Article31. Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009; 9:407–416. PMID: 19416711.
Article32. Fu A, Eberhard CE, Screaton RA. Role of AMPK in pancreatic beta cell function. Mol Cell Endocrinol. 2013; 366:127–134. PMID: 22766107.
Article33. Henriksen BS, Curtis ME, Fillmore N, Cardon BR, Thomson DM, Hancock CR. The effects of chronic AMPK activation on hepatic triglyceride accumulation and glycerol 3-phosphate acyltransferase activity with high fat feeding. Diabetol Metab Syndr. 2013; 5:29. PMID: 23725555.
Article34. Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004; 279:47898–47905. PMID: 15371448.
Article35. Unger RH. How obesity causes diabetes in Zucker diabetic fatty rats. Trends Endocrinol Metab. 1997; 8:276–282. PMID: 18406816.
Article36. Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, Mauvais-Jarvis F. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Invest. 2011; 121:3331–3342. PMID: 21747171.
Article37. Dai YL, Huang SL, Leng Y. AICAR and metformin exert AMPK-dependent effects on INS-1E pancreatic β-cell apoptosis via differential downstream mechanisms. Int J Biol Sci. 2015; 11:1272–1280. PMID: 26435693.
Article38. Tao W, Wu J, Xie BX, Zhao YY, Shen N, Jiang S, Wang XX, Xu N, Jiang C, Chen S, Gao X, Xue B, Li CJ. Lipid-induced muscle insulin resistance is mediated by GGPPS via modulation of the RhoA/Rho kinase signaling pathway. J Biol Chem. 2015; 290:20086–20097. PMID: 26112408.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Perspective of Metformin
- Rethinking about Metformin: Promising Potentials
- Anticancer effect of metformin alone and in combination with 2-deoxy-D-glucose on mouse T cell lymphoma EL4 cells
- Metformin Based Dual-Combination Therapies in Drug Naive Type 2 Diabetic Patients
- Metformin Suppresses MHC-Restricted Antigen Presentation by Inhibiting Co-Stimulatory Factors and MHC Molecules in APCs