Diabetes Metab J.
2019 Oct;43(5):683-699. 10.4093/dmj.2019.0112.
PF-04620110, a Potent Antidiabetic Agent, Suppresses Fatty Acid-Induced NLRP3 Inflammasome Activation in Macrophages
- Affiliations
-
- 1Department of Integrated Biomedical Science, Soonchunhyang Institute of Medi-bio Science (SIMS), Soonchunhyang University, Cheonan, Korea. jongseok81@sch.ac.kr
- KMID: 2460961
- DOI: http://doi.org/10.4093/dmj.2019.0112
Abstract
- BACKGROUND
Chronic inflammation has been linked to insulin resistance and type 2 diabetes mellitus (T2DM). High-fat diet (HFD)-derived fatty acid is associated with the activation of chronic inflammation in T2DM. PF-04620110, which is currently in phase 1 clinical trials as a selective acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) inhibitor, is a potent anti-diabetic agent that may be important for the regulation of chronic inflammation in T2DM. However, the mechanisms by which PF-04620110 regulates fatty acid-induced chronic inflammation remain unclear.
METHODS
PF-04620110 was used in vitro and in vivo. DGAT1-targeting gRNAs were used for deletion of mouse DGAT1 via CRISPR ribonucleoprotein (RNP) system. The activation of NLRP3 inflammasome was measured by immunoblot or cytokine analysis in vitro and in vivo.
RESULTS
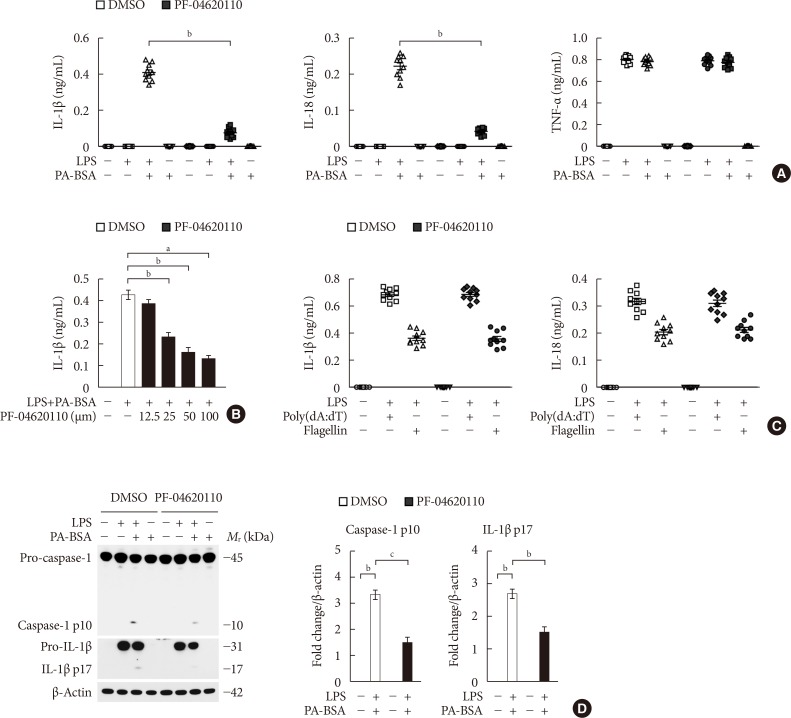
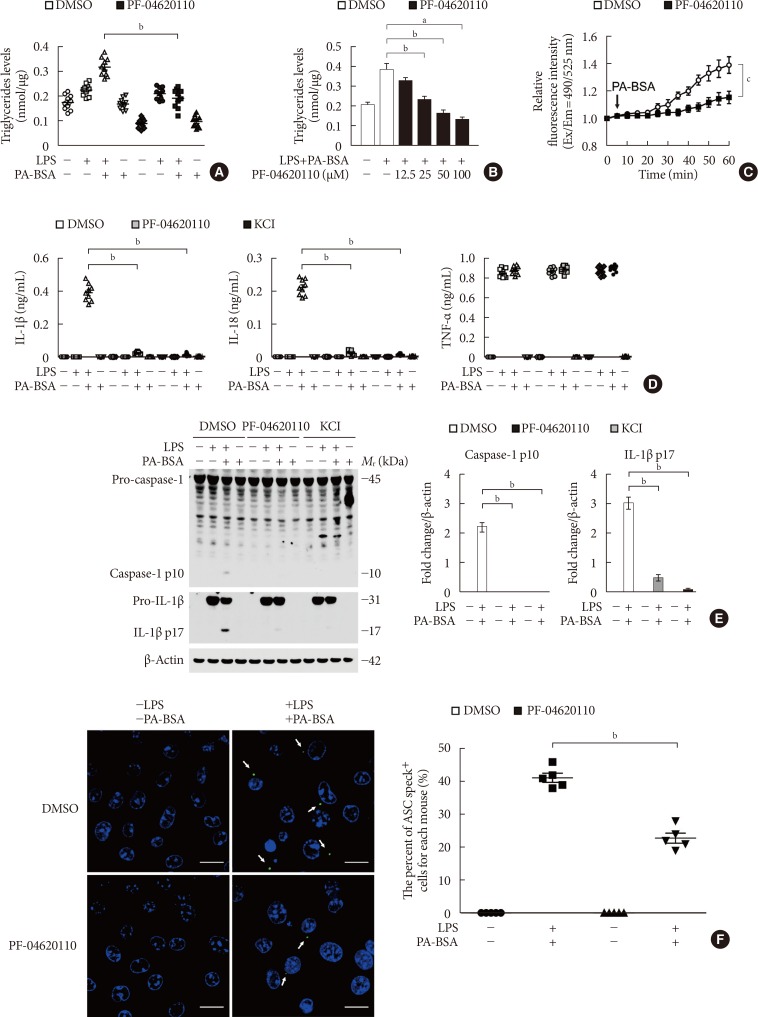
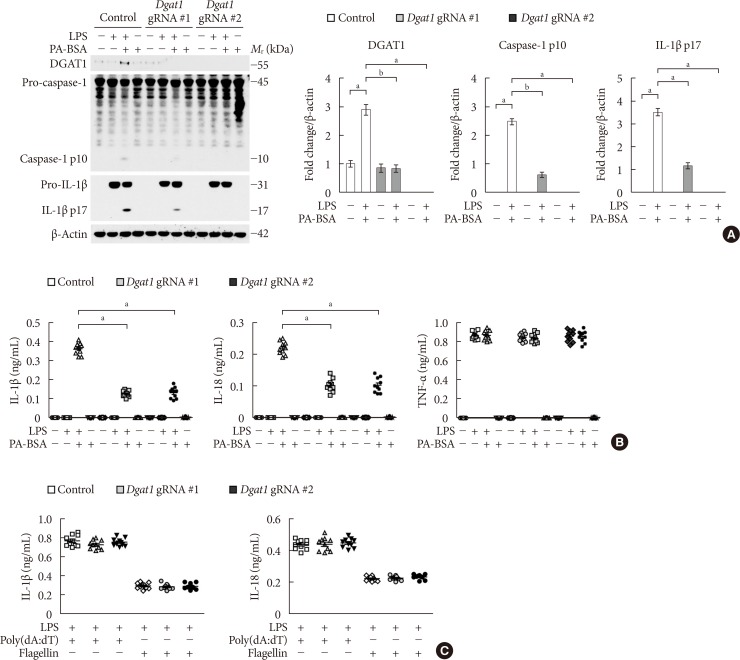
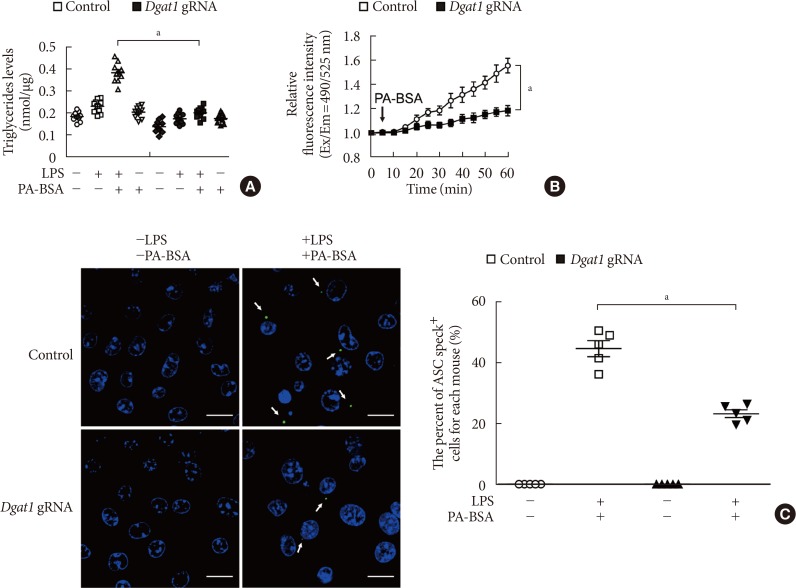
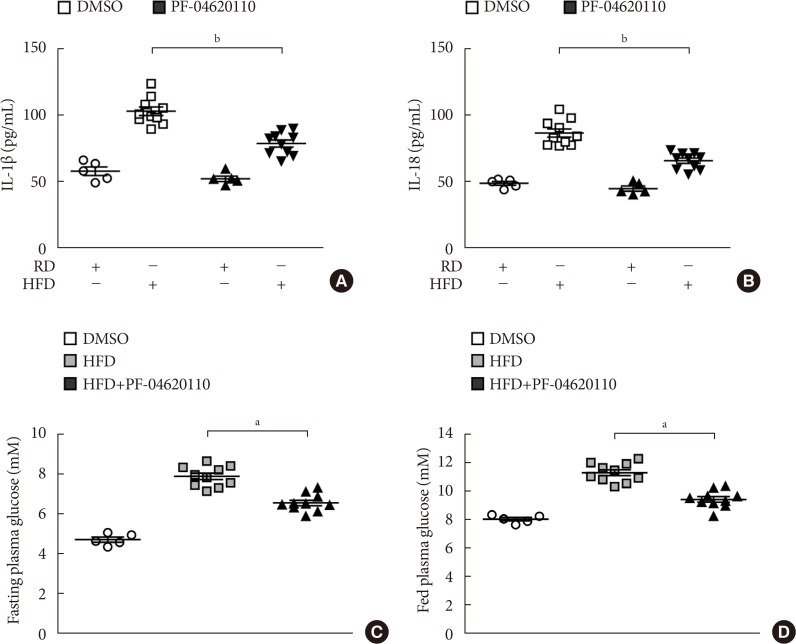
Here we show that PF-04620110 suppressed fatty acid-induced nucleotide-binding domain, leucine-rich-repeat-containing receptor (NLR), pyrin-domain-containing 3 (NLRP3) inflammasome activation in macrophages. In contrast, PF-04620110 did not change the activation of the NLR family, CARD-domain-containing 4 (NLRC4), or the absent in melanoma 2 (AIM2) inflammasomes. Moreover, PF-04620110 inhibited K⺠efflux and the NLRP3 inflammasome complex formation, which are required for NLRP3 inflammasome activation. PF-04620110 reduced the production of interleukin 1β (IL-1β) and IL-18 and blood glucose levels in the plasma of mice fed HFD. Furthermore, genetic inhibition of DGAT1 suppressed fatty acid-induced NLRP3 inflammasome activation.
CONCLUSION
Our results suggest that PF-04620110 suppresses fatty acid-induced NLRP3 inflammasome activation.
Keyword
MeSH Terms
-
Animals
Blood Glucose
Clinical Trials, Phase I as Topic
Clustered Regularly Interspaced Short Palindromic Repeats
Diabetes Mellitus, Type 2
Diacylglycerol O-Acyltransferase
Diet, High-Fat
Fatty Acids
Humans
In Vitro Techniques
Inflammasomes*
Inflammation
Insulin Resistance
Interleukin-18
Interleukins
Macrophages*
Melanoma
Mice
Plasma
Ribonucleoproteins
RNA, Guide
Blood Glucose
Diacylglycerol O-Acyltransferase
Fatty Acids
Inflammasomes
Interleukin-18
Interleukins
RNA, Guide
Ribonucleoproteins
Figure
Reference
-
1. Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009; 10:241–247. PMID: 19221555.
Article2. Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006; 24:317–327. PMID: 16546100.
Article3. Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010; 10:210–215. PMID: 20168318.
Article4. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013; 13:397–411. PMID: 23702978.
Article5. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867. PMID: 17167474.
Article6. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011; 12:408–415. PMID: 21478880.
Article7. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015; 21:263–269. PMID: 25686106.
Article8. Moon JS, Hisata S, Park MA, DeNicola GM, Ryter SW, Nakahira K, Choi AMK. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Rep. 2015; 12:102–115. PMID: 26119735.
Article9. Moon JS, Nakahira K, Chung KP, DeNicola GM, Koo MJ, Pabon MA, Rooney KT, Yoon JH, Ryter SW, Stout-Delgado H, Choi AM. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med. 2016; 22:1002–1012. PMID: 27455510.
Article10. Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003; 52:812–817. PMID: 12606524.11. Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996; 39:1005–1029. PMID: 8877284.
Article12. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002; 110:851–860. PMID: 12235117.13. Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007; 148:241–251. PMID: 17038556.14. Lagathu C, Yvan-Charvet L, Bastard JP, Maachi M, Quignard-Boulange A, Capeau J, Caron M. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 2006; 49:2162–2173. PMID: 16865359.15. Boden G. Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care. 2002; 5:545–549. PMID: 12172479.
Article16. Dow RL, Li JC, Pence MP, Gibbs EM, LaPerle JL, Litchfield J, Piotrowski DW, Munchhof MJ, Manion TB, Zavadoski WJ, Walker GS, McPherson RK, Tapley S, Sugarman E, Guzman-Perez A, DaSilva-Jardine P. Discovery of PF-04620110, a potent, selective, and orally bioavailable inhibitor of DGAT-1. ACS Med Chem Lett. 2011; 2:407–412. PMID: 24900321.
Article17. Enayetallah AE, Ziemek D, Leininger MT, Randhawa R, Yang J, Manion TB, Mather DE, Zavadoski WJ, Kuhn M, Treadway JL, des Etages SA, Gibbs EM, Greene N, Steppan CM. Modeling the mechanism of action of a DGAT1 inhibitor using a causal reasoning platform. PLoS One. 2011; 6:e27009. PMID: 22073239.
Article18. King AJ, Segreti JA, Larson KJ, Souers AJ, Kym PR, Reilly RM, Zhao G, Mittelstadt SW, Cox BF. Diacylglycerol acyltransferase 1 inhibition lowers serum triglycerides in the Zucker fatty rat and the hyperlipidemic hamster. J Pharmacol Exp Ther. 2009; 330:526–531. PMID: 19478132.
Article19. Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014; 156:1193–1206. PMID: 24630722.
Article20. Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J, Fernandes-Alnemri T, Alnemri ES. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006; 13:236–249. PMID: 16037825.21. Horwood NJ, Mahon T, McDaid JP, Campbell J, Mano H, Brennan FM, Webster D, Foxwell BM. Bruton's tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production. J Exp Med. 2003; 197:1603–1611. PMID: 12810683.22. Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, Brennan FM, Webster D, Foxwell BM. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol. 2006; 176:3635–3641. PMID: 16517732.
Article24. Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015; 21:248–255. PMID: 25686105.
Article25. Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson AAB, Butler MS, Rowe DB, O'Neill LA, Kanthasamy AG, Schroder K, Cooper MA, Woodruff TM. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018; 10:eaah4066. PMID: 30381407.
Article26. Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A. 2007; 104:8041–8046. PMID: 17483456.
Article27. Marchetti C, Swartzwelter B, Koenders MI, Azam T, Tengesdal IW, Powers N, de Graaf DM, Dinarello CA, Joosten LAB. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res Ther. 2018; 20:169. PMID: 30075804.
Article28. Huang Y, Jiang H, Chen Y, Wang X, Yang Y, Tao J, Deng X, Liang G, Zhang H, Jiang W, Zhou R. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. 2018; 10:e8689. PMID: 29531021.
Article29. He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, Liu Q, Liang G, Deng X, Jiang W, Zhou R. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018; 9:2550. PMID: 29959312.
Article30. Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci U S A. 1998; 95:13018–13023. PMID: 9789033.
Article31. Cao J, Zhou Y, Peng H, Huang X, Stahler S, Suri V, Qadri A, Gareski T, Jones J, Hahm S, Perreault M, McKew J, Shi M, Xu X, Tobin JF, Gimeno RE. Targeting Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. J Biol Chem. 2011; 286:41838–41851. PMID: 21990351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout
- Ethanol Augments Monosodium Urate-Induced NLRP3 Inflammasome Activation via Regulation of AhR and TXNIP in Human Macrophages
- Loganin Prevents Hepatic Steatosis by Blocking NLRP3 Inflammasome Activation
- Repurposing Auranofin, an Anti-Rheumatic Gold Compound, to Treat Acne Vulgaris by Targeting the NLRP3 Inflammasome
- Cobalt Chloride-induced Hypoxia Ameliorates NLRP3-Mediated Caspase-1 Activation in Mixed Glial Cultures