J Breast Cancer.
2018 Mar;21(1):28-36. 10.4048/jbc.2018.21.1.28.
Troglitazone Inhibits Matrix Metalloproteinase-9 Expression and Invasion of Breast Cancer Cell through a Peroxisome Proliferator-Activated Receptor γ-Dependent Mechanism
- Affiliations
-
- 1Department of Biochemistry, Institute for Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea. jsukim@jbnu.ac.kr
- 2Department of Surgery, Research Institute of Clinical Medicine, Chonbuk National University Hospital, Chonbuk National University and Biomedical Research Institute, Jeonju, Korea.
- 3Department of Oral Biochemistry and Institute of Biomaterials, Implant, School of Dentistry, Wonkwang University College of Medicine, Iksan, Korea.
- 4Department of Obstetrics Gynecology, Chonbuk National University Medical School, Jeonju, Korea.
- 5Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 6Department of Glycobiology, Institute of Biological Science, Sungkyunkwan University, Suwon, Korea.
- KMID: 2441865
- DOI: http://doi.org/10.4048/jbc.2018.21.1.28
Abstract
- PURPOSE
Peroxisome proliferator-activated receptor γ (PPARγ) is involved in the pathology of numerous diseases including atherosclerosis, diabetes, obesity, and cancer. Matrix metalloproteinases (MMPs) play a significant role in tissue remodeling related to various processes such as morphogenesis, angiogenesis, tissue repair, invasion, and metastasis. We investigated the effects of PPARγ on MMP expression and invasion in breast cancer cells.
METHODS
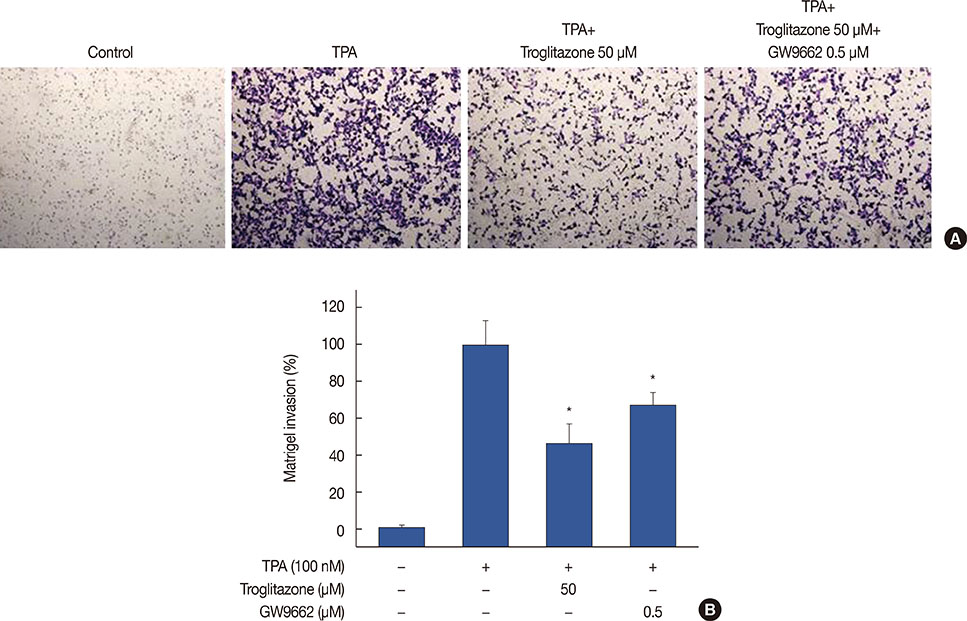
MCF-7 cells were cultured and then cell viability was monitored in an MTT assay. Western blotting, gelatin zymography, real-time polymerase chain reaction, and luciferase assays were performed to investigate the effect of the synthetic PPARγ ligand troglitazone on MMP expression. Transcription factor DNA binding was analyzed by electrophoretic mobility shift assay. A Matrigel invasion assay was used to assess the effects of troglitazone on MCF-7 cells.
RESULTS
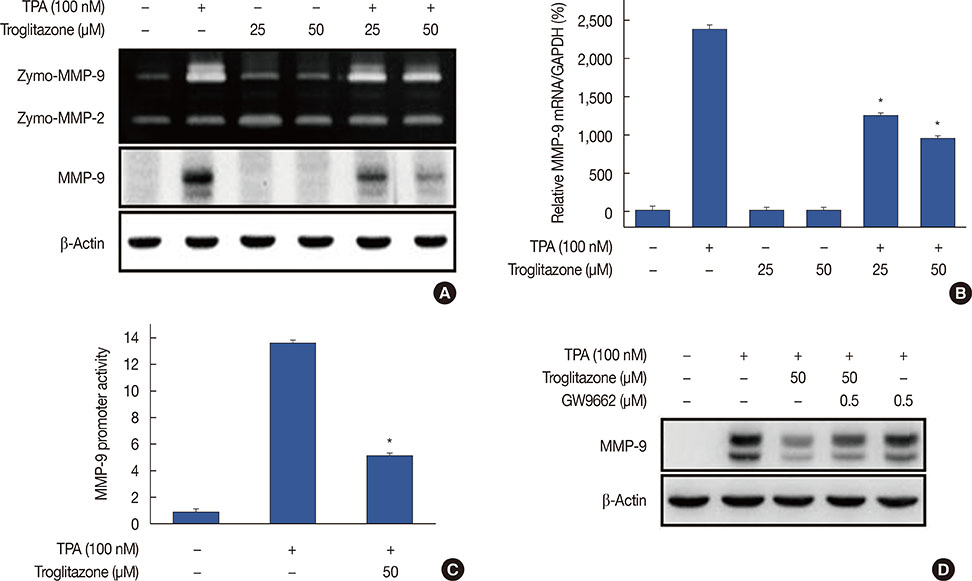
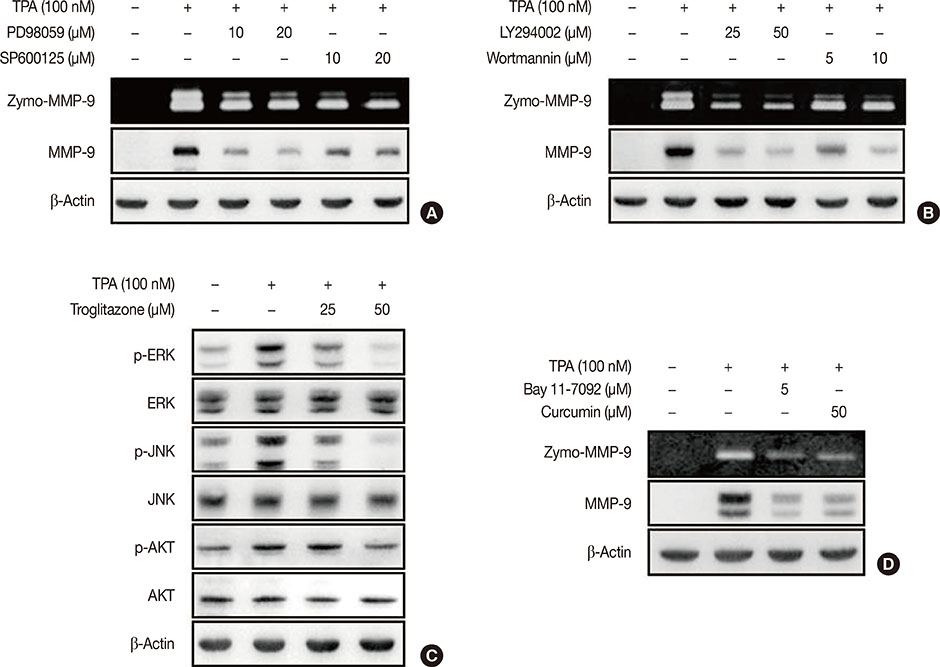
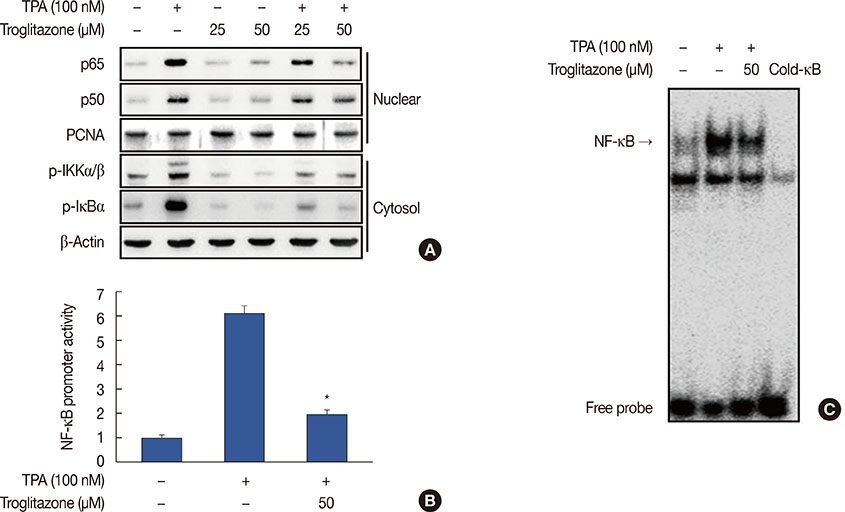
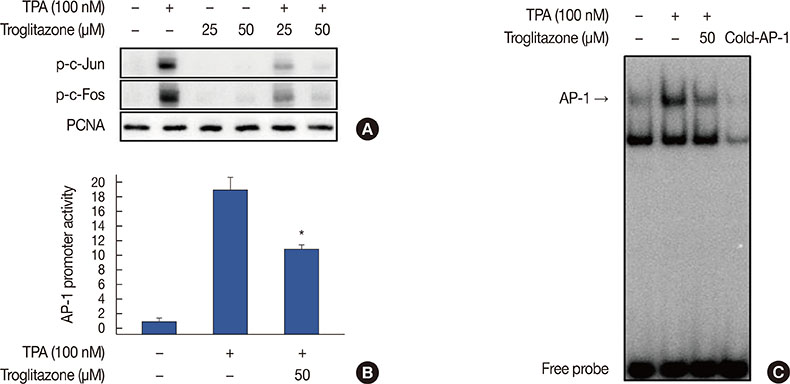
Troglitazone did not affect MCF-7 cell viability. 12-O-tetradecanoylphorbol-13-acetate (TPA) induced MMP-9 expression and invasion in MCF-7 cell. However, these effects were decreased by troglitazone. TPA increased nuclear factor κB and activator protein-1 DNA binding, while troglitazone inhibited these effects. The selective PPARγ antagonist GW9662 reversed MMP-9 inhibition by troglitazone in TPA-treated MCF-7 cells.
CONCLUSION
Troglitazone inhibited nuclear factor κB and activator protein-1-mediated MMP-9 expression and invasion of MCF-7 cells through a PPARγ-dependent mechanism.
MeSH Terms
-
Atherosclerosis
Blotting, Western
Breast Neoplasms*
Breast*
Cell Survival
DNA
Electrophoretic Mobility Shift Assay
Gelatin
Luciferases
Matrix Metalloproteinase 9*
Matrix Metalloproteinases
MCF-7 Cells
Morphogenesis
Neoplasm Metastasis
NF-kappa B
Obesity
Pathology
Peroxisomes*
PPAR gamma
Real-Time Polymerase Chain Reaction
Transcription Factor AP-1
Transcription Factors
DNA
Gelatin
Luciferases
Matrix Metalloproteinase 9
Matrix Metalloproteinases
NF-kappa B
PPAR gamma
Transcription Factor AP-1
Transcription Factors
Figure
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article2. Takaoka M, Naomoto Y, Ohkawa T, Uetsuka H, Shirakawa Y, Uno F, et al. Heparanase expression correlates with invasion and poor prognosis in gastric cancers. Lab Invest. 2003; 83:613–622.
Article3. Wang L, Ling Y, Chen Y, Li CL, Feng F, You QD, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010; 297:42–48.
Article4. Yao J, Xiong S, Klos K, Nguyen N, Grijalva R, Li P, et al. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene. 2001; 20:8066–8074.
Article5. Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002; 21:251–262.
Article6. Lee WT, Lee TH, Cheng CH, Chen KC, Chen YC, Lin CW. Antroquinonol from Antrodia Camphorata suppresses breast tumor migration/invasion through inhibiting ERK-AP-1- and AKT-NF-kappaB-dependent MMP-9 and epithelial-mesenchymal transition expressions. Food Chem Toxicol. 2015; 78:33–41.
Article7. Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996; 37:907–925.
Article8. Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008; 456:350–356.
Article9. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002; 53:409–435.
Article10. Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr Metab (Lond). 2005; 2:30.
Article11. Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008; 134:703–707.
Article12. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011; 11:85–95.
Article13. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E745–E751.
Article14. Allred CD, Kilgore MW. Selective activation of PPARgamma in breast, colon, and lung cancer cell lines. Mol Cell Endocrinol. 2005; 235:21–29.
Article15. Fenner MH, Elstner E. Peroxisome proliferator-activated receptor-gamma ligands for the treatment of breast cancer. Expert Opin Investig Drugs. 2005; 14:557–568.
Article16. Yin Y, Russell RG, Dettin LE, Bai R, Wei ZL, Kozikowski AP, et al. Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res. 2005; 65:3950–3957.
Article17. Elstner E, Müller C, Koshizuka K, Williamson EA, Park D, Asou H, et al. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci U S A. 1998; 95:8806–8811.
Article18. Yu J, Qiao L, Zimmermann L, Ebert MP, Zhang H, Lin W, et al. Troglitazone inhibits tumor growth in hepatocellular carcinoma in vitro and in vivo. Hepatology. 2006; 43:134–143.
Article19. Yu HN, Lee YR, Noh EM, Lee KS, Kim JS, Song EK, et al. Induction of G1 phase arrest and apoptosis in MDA-MB-231 breast cancer cells by troglitazone, a synthetic peroxisome proliferator-activated receptor gamma (PPARgamma) ligand. Cell Biol Int. 2008; 32:906–912.
Article20. Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998; 435:29–34.
Article21. Chiang PC, Lin SC, Pan SL, Kuo CH, Tsai IL, Kuo MT, et al. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: a crucial role of AMPK and mTOR pathways. Biochem Pharmacol. 2010; 79:162–171.
Article22. Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, et al. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 2011; 55:594–605.
Article23. Yang YT, Weng CJ, Ho CT, Yen GC. Resveratrol analog-3,5,4’-trimethoxy-trans-stilbene inhibits invasion of human lung adenocarcinoma cells by suppressing the MAPK pathway and decreasing matrix metalloproteinase-2 expression. Mol Nutr Food Res. 2009; 53:407–416.
Article24. Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999; 13:781–792.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peroxisome proliferator-activated receptor gamma activator inhibits cell growth of MDA-MB-231 breast cancer cells through induction of apoptosis
- Effect of Curcumin on Cancer Invasion and Matrix Metalloproteinase-9 Activity in MDA-MB-231 Human Breast Cancer Cell
- Peroxisome Proliferator-Activated Receptor gammaActivation Promotes Adipogenesis in Human Mesenchymal Stem Cells
- Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway
- Redifferentiation Effects of Troglitazone in Human Thyroid Cancer Cell Lines