J Breast Cancer.
2006 Dec;9(4):293-300. 10.4048/jbc.2006.9.4.293.
Peroxisome proliferator-activated receptor gamma activator inhibits cell growth of MDA-MB-231 breast cancer cells through induction of apoptosis
- Affiliations
-
- 1Division of Breast and Endocrine Surgery, Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea. shjung@chonbuk.ac.kr
- KMID: 2286600
- DOI: http://doi.org/10.4048/jbc.2006.9.4.293
Abstract
-
PURPOSE: Peroxisome proliferator-activated receptor gamma (PPARgamma) has become a potential target for the prevention and treatment of human cancers. PPARgamma ligands inhibit cell proliferation of estrogen receptoralpha(ERalpha)-positive breast cancer cells. However, it has recently been shown that ERalpha-negatively inhibits PPARgamma signaling in breast cancer cells, indicating that PPARgamma ligand may be more useful for treating ERalpha-negative breast cancer cells compared to ERalpha-positive breast cancer cells. In this study, we attempted to elucidate the role of PPARg in ERalpha-negative breast cancer cells.
METHODS
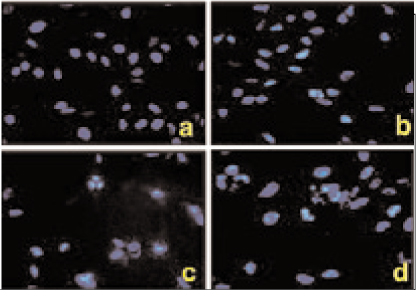
The effect of PPARgamma ligand on the growth of MDA-MB-231 cells was measured by MTT assay and flow cytometric analysis. TUNEL staining and Hoechst 33342 fluorescent staining were used to observe the effects of PPARgamma ligand on cell apoptosis. The regulatory proteins of the cell cycle were measured by Western blot.
RESULTS
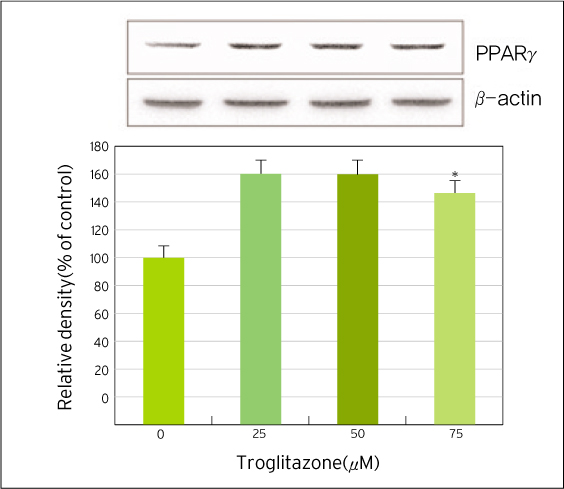
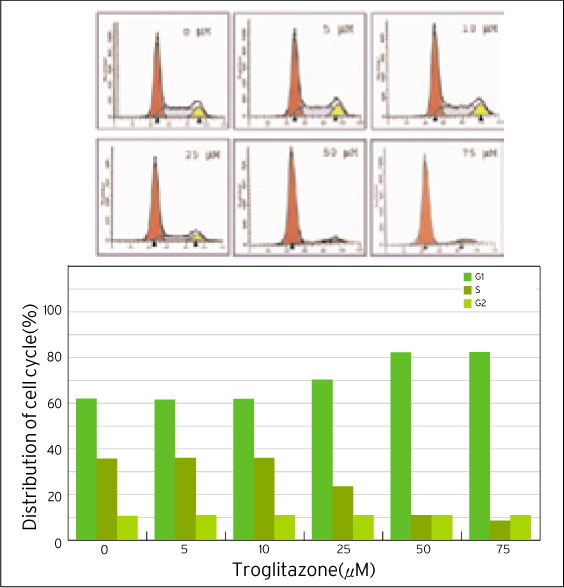
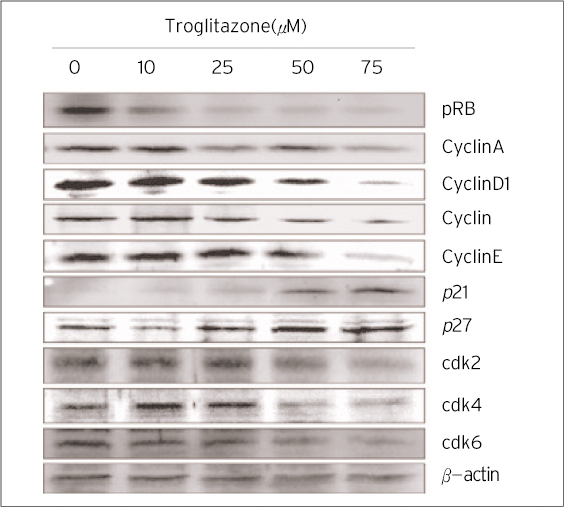
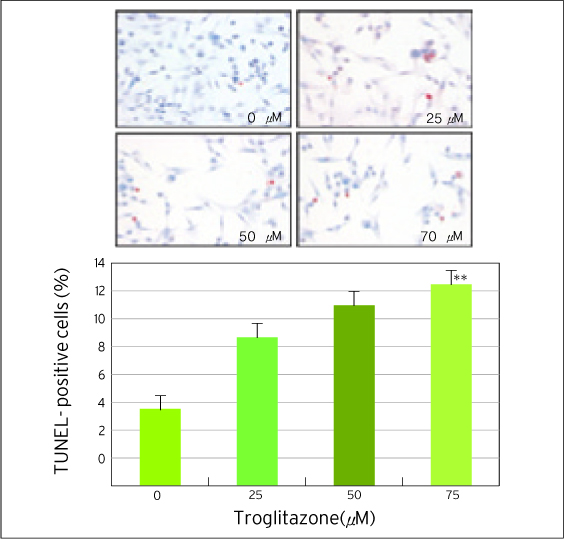
The treatment of MDA-MB-231 human breast cancer cells with the PPARgamma ligand, trgoglitazone, was shown to induce inhibition of cell growth in a dose-dependent manner. Cell cycle analysis showed a G1 arrest in MDA-MB-231 cells exposed to troglitazone. The apoptotic effect by troglitazone demonstrated that apoptotic cells were elevated from 2.5-fold of the control level at 10 mM, to 3.1-fold at 50micrometer and to 3.5-fold at 75 mM of troglitazone. Moreover, troglitazone treatment dose-dependently caused a marked decrease in the pRb, cyclin D1, cyclin D2, cyclin D3, cdk2, Cdk4 and Cdk6 expressions and there was a significant increase in the p21 and p27 expressions.
CONCLUSION
These results indicate that trgoglitazone induces cell-cycle G1 arrest and apoptosis in ERalpha-negative MDA-MB-231 breast cancer cells. Collectively, this paper shows that PPARgamma ligand is an important player as a member of the chemotherapeutic candidates for treating ERalpha-negative breast cancer.
MeSH Terms
Figure
Reference
-
1. Houssami N, Cuzick J, Dixon JM. The prevention, detection, and management of breast cancer. Med J Aust. 2006. 184:230–234.
Article2. Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004. 96:621–628.
Article3. Muss HB. Endocrine therapy for advanced breast cancer: a review. Breast Cancer Res Treat. 1992. 2:15–26.
Article4. Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma: tissue specific regulator of an adipocyte enhancer. Genes Dev. 1994. 8:1224–1234.5. Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006. 86:465–514.
Article6. Kletzien RF, Clarke SD, Ulrich RG. Enhancement of adipocyte differentiation by an insulin-sensitizing agent. Mol Pharmacol. 1992. 41:393–398.7. Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, et al. Effect of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc Natl Acad Sci. 2000. 97:10990–10995.
Article8. Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992. 68:879–887.
Article9. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferator. Nature. 1990. 347:645–650.
Article10. Kliewer MW, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci. 1994. 91:7355–7359.
Article11. Panigraphy D, Huang S, Kieran MW, Kaipainen A. PPARgamma as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biol Ther. 2005. 4:687–693.12. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995. 83:803–812.
Article13. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci. 1997. 94:4318–4323.
Article14. Larsen TM, Toubro S, Astrup S. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disor. 2003. 27:147–161.
Article15. McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci. 2003. 100:131–136.
Article16. Thoennes SR, Tate PL, Price TM, Kilgore MW. Differential transcriptional activation of peroxisome proliferatoractivated receptor gamma by omega-3 and omega-6 fatty acids in MCF-7 cells. Mol Cell Endocrinol. 2000. 160:67–73.
Article17. Allred CD, Kilgore MW. Selective activation of PPARγ in breast, colon, and lung cancer cell lines. Mol Cell Endocrinol. 2005. 235:21–29.
Article18. Semple RK, Chatterjee VK, O'Rahilly S. PPARγ and human metabolic disease. J Clin Invest. 2006. 116:581–589.
Article19. Yin F, Wahino S, Liu Z, Kim S, Hsueh WA, Collins AR, et al. Triglitazone inhibits growth of MCF-7 breast cancer carcinoma cells by targeting G1 cell cycle regulators. Biochem Biophys Res Commun. 2001. 286:916–922.
Article20. Ferner MH, Elstner E. Peroxisome proliferator-activated receptor gamma ligands for the treatment of breast cancer. Expert Opin Investig Drugs. 2005. 14:557–568.21. Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, et al. Estrogen receptorγ binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptorγ signaling in breast cancer cells. Human Cancer Biol. 2005. 11:6139–6147.
Article22. Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP. Use of the peroxisome proliferator-activated receptor (PPARγ) ligand, troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res Treat. 2003. 79:391–397.
Article23. Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, et al. Ligands for peroxisome proliferator-activated receptor gamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci. 1998. 95:8806–8811.
Article24. Yu J, Qiao L, Zimmermann L, Ebert MP, Zhang H, Lin W, et al. Troglitazone inhibits tumor growth in hepatocellular carcinoma in vitro and in vivo. Hepatology. 2006. 43:134–143.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines
- The anti-cancer effect of pomegranate-derived nanovesicles on MDA-MB-231 breast cancer cells
- Effect of corosolic acid on apoptosis and angiogenesis in MDA-MB-231 human breast cancer cells
- Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells
- Effect of Curcumin on Cancer Invasion and Matrix Metalloproteinase-9 Activity in MDA-MB-231 Human Breast Cancer Cell