Nutr Res Pract.
2016 Oct;10(5):494-500. 10.4162/nrp.2016.10.5.494.
Blueberry, blackberry, and blackcurrant differentially affect plasma lipids and pro-inflammatory markers in diet-induced obesity mice
- Affiliations
-
- 1Department of Nutritional Sciences, University of Connecticut, Storrs, CT 06269, USA. ji-young.lee@uconn.edu
- KMID: 2434083
- DOI: http://doi.org/10.4162/nrp.2016.10.5.494
Abstract
- BACKGROUND/OBJECTIVES
Evidence indicates that berry anthocyanins are anti-atherogenic, antioxidant, and anti-inflammatory. However, berries differ vastly in their anthocyanin composition and thus potentially in their biological and metabolic effects. The present study compared hypolipidemic, antioxidant, and anti-inflammatory properties of blueberry (BB), blackberry (BK), and blackcurrant (BC) in a diet-induced obesity (DIO) mouse model.
MATERIALS/METHODS
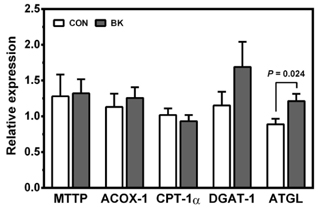
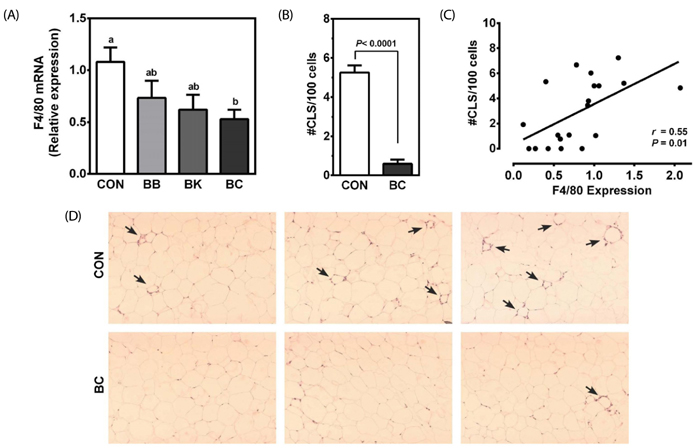
Male C57BL/6J mice were fed a high fat (HF; 35% fat, w/w) control diet or a HF diet supplemented with freeze-dried 5% BB, 6.3% BK or 5.7% BC for 12 weeks (10 mice/group) to achieve the same total anthocyanin content in each diet. Plasma lipids, antioxidant status and pro-inflammatory cytokines were measured. The expression of genes involved in antioxidant defense, inflammation, and lipid metabolism was determined in the liver, epididymal adipose tissue, proximal intestine, and skeletal muscle. Histological analysis was performed to identify crown-like structure (CLS) in epididymal fat pads to determine macrophage infiltration.
RESULTS
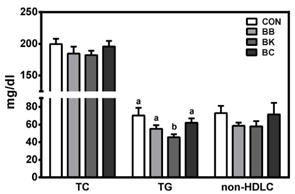
No differences were noted between the control and any berry-fed groups in plasma levels of liver enzymes, insulin, glucose, ferric reducing antioxidant power, superoxide dismutase, and tumor necrosis factor α. However, BK significantly lowered plasma triglyceride compared with the HF control and other berries, whereas BC significantly reduced F4/80 mRNA and the number of CLS in the epididymal fat pad, indicative of less macrophage infiltration.
CONCLUSIONS
The present study provides evidence that BB, BK and BC with varying anthocyanin composition differentially affect plasma lipids and adipose macrophage infiltration in DIO mice, but with no differences in their antioxidant capacity and anti-inflammatory potential.
MeSH Terms
-
Adipose Tissue
Animals
Anthocyanins
Blueberry Plant*
Cytokines
Diet
Fruit
Glucose
Humans
Inflammation
Insulin
Intestines
Lipid Metabolism
Liver
Macrophages
Male
Mice*
Muscle, Skeletal
Obesity*
Plasma*
RNA, Messenger
Rubus*
Superoxide Dismutase
Triglycerides
Tumor Necrosis Factor-alpha
Anthocyanins
Cytokines
Glucose
Insulin
RNA, Messenger
Superoxide Dismutase
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Delphinidin enhances radio-therapeutic effects via autophagy induction and JNK/MAPK pathway activation in non-small cell lung cancer
Seong Hee Kang, Dong-Ho Bak, Byung Yeoup Chung, Hyoung-Woo Bai, Bo Sun Kang
Korean J Physiol Pharmacol. 2020;24(5):413-422. doi: 10.4196/kjpp.2020.24.5.413.
Reference
-
1. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. American Heart Association. Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006; 113:898–918.
Article2. Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010; 2010:535918.
Article3. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006; 26:968–976.
Article4. Mathieu P, Poirier P, Pibarot P, Lemieux I, Després JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009; 53:577–584.5. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006; 444:875–880.
Article6. Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H, Schulze M, Stehle P, Watzl B. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012; 51:637–663.
Article7. Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001; 134:1106–1114.
Article8. Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, Hong CP, Sinaiko AR. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009; 109:414–421.
Article9. Bazzano LA, Serdula MK, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003; 5:492–499.
Article10. Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006; 54:4069–4075.
Article11. Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007; 51:675–683.
Article12. Prior RL, Wilkes SE, Rogers TR, Khanal RC, Wu X, Howard LR. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010; 58:3970–3976.
Article13. Riso P, Klimis-Zacas D, Del Bo' C, Martini D, Campolo J, Vendrame S, Møller P, Loft S, De Maria R, Porrini M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. 2013; 52:949–961.
Article14. Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E, Dugo G, Caputi AP, Cuzzocrea S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003; 73:1097–1114.
Article15. Gopalan A, Reuben SC, Ahmed S, Darvesh AS, Hohmann J, Bishayee A. The health benefits of blackcurrants. Food Funct. 2012; 3:795–809.
Article16. He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. 2010; 1:163–187.
Article17. Chang HJ, Choi EH, Chun HS. Quantitative structure-activity relationship (QSAR) of antioxidative anthocyanidins and their glycosides. Food Sci Biotechnol. 2008; 17:501–507.18. Hou DX, Yanagita T, Uto T, Masuzaki S, Fujii M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: structure-activity relationship and molecular mechanisms involved. Biochem Pharmacol. 2005; 70:417–425.
Article19. Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Current Protocols in Food Analytical Chemistry. Hoboken (NJ): John Wiley & Sons, Inc.;2001. p. F1.2.1–F1.2.13.20. Wu X, Prior RL. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J Agric Food Chem. 2005; 53:2589–2599.
Article21. Kim B, Ku CS, Pham TX, Park Y, Martin DA, Xie L, Taheri R, Lee J, Bolling BW. Aronia melanocarpa (chokeberry) polyphenol-rich extract improves antioxidant function and reduces total plasma cholesterol in apolipoprotein E knockout mice. Nutr Res. 2013; 33:406–413.
Article22. Yang Y, Seo JM, Nguyen A, Pham TX, Park HJ, Park Y, Kim B, Bruno RS, Lee J. Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J Nutr. 2011; 141:1611–1617.
Article23. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996; 239:70–76.
Article24. Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993; 26:39–42.
Article25. Kim B, Park Y, Wegner CJ, Bolling BW, Lee J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells. J Nutr Biochem. 2013; 24:1564–1570.
Article26. Ku CS, Pham TX, Park Y, Kim B, Shin MS, Kang I, Lee J. Edible blue-green algae reduce the production of pro-inflammatory cytokines by inhibiting NF-kappaB pathway in macrophages and splenocytes. Biochim Biophys Acta. 2013; 1830:2981–2988.
Article27. Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011; 51:1106–1115.
Article28. Lee SG, Kim B, Yang Y, Pham TX, Park YK, Manatou J, Koo SI, Chun OK, Lee JY. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J Nutr Biochem. 2014; 25:404–411.
Article29. Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med. 2010; 31:446–467.
Article30. Ferreira de Araujo PR, da Silva Santos V, Rodrigues Machado A, Gevehr Fernandes C, Silva JA, da Silva Rodrigues R. Benefits of blackberry nectar (Rubus spp.) relative to hypercholesterolemia and lipid peroxidation. Nutr Hosp. 2011; 26:984–990.31. Prior RL, Wu X, Gu L, Hager T, Hager A, Wilkes S, Howard L. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol Nutr Food Res. 2009; 53:1406–1418.
Article32. Perseghin G. Muscle lipid metabolism in the metabolic syndrome. Curr Opin Lipidol. 2005; 16:416–420.
Article33. Jia Y, Kim JY, Jun HJ, Kim SJ, Lee JH, Hoang MH, Kim HS, Chang HI, Hwang KY, Um SJ, Lee SJ. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim Biophys Acta. 2012; 1831:698–708.
Article34. Um MY, Ahn J, Ha TY. Hypolipidaemic effects of cyanidin 3-glucoside rich extract from black rice through regulating hepatic lipogenic enzyme activities. J Sci Food Agric. 2013; 93:3126–3128.
Article35. Wei X, Wang D, Yang Y, Xia M, Li D, Li G, Zhu Y, Xiao Y, Ling W. Cyanidin-3-O-beta-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J Sci Food Agric. 2011; 91:1006–1013.
Article36. Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007; 137:2043–2048.
Article37. Lin CY, Huang CS, Huang CY, Yin MC. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J Agric Food Chem. 2009; 57:6661–6667.
Article38. Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008; 117:798–805.
Article39. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010; 17:332–341.
Article40. Wajchenberg BL, Nery M, Cunha MR, Silva ME. Adipose tissue at the crossroads in the development of the metabolic syndrome, inflammation and atherosclerosis. Arq Bras Endocrinol Metabol. 2009; 53:145–150.
Article41. Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007; 56:564–573.
Article42. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808.
Article43. Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014; 19:162–171.
Article44. Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, Li D, Zhang Y, Li G, Xiao Y, Liu F, Li Z, Shi Z, Yang Y. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. 2013; 23:843–849.
Article45. Prior RL, Wu X. Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res. 2006; 40:1014–1028.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of fermented blueberry liquid in high-fat diet-induced obese C57BL/6J mice
- Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice
- Impact of High Fat Diet-induced Obesity on the Plasma Levels of Monoamine Neurotransmitters in C57BL/6 Mice
- Vitamin D Attenuates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obesity Murine Model
- The Inhibitory Effect of Quercetin on Adipose Tissue Inflammation in Mice Fed on a High-fat Diet