Nutr Res Pract.
2016 Feb;10(1):42-48. 10.4162/nrp.2016.10.1.42.
Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Jeju National University, 102 Jejudaehak-ro, Jeju 63243, Korea. lyk1230@jejunu.ac.kr
- 2College of Veterinary Medicine, Jeju National University, 102 Jejudaehak-ro, Jeju, 63243, Korea.
- KMID: 2313899
- DOI: http://doi.org/10.4162/nrp.2016.10.1.42
Abstract
- BACKGROUND/OBJECTIVES
Seaweeds have been reported to have various health beneficial effects. In this study, we investigated the potential anti-obesity and anti-inflammatory effects of four types of domestic brown seaweeds in a high-fat diet-induced obese mouse model and bone marrow-derived macrophages (BMDM).
MATERIALS/METHODS
Male C57BL/6N mice were fed low-fat diet (LFD), high-fat diet (HFD) or HFD containing Undaria Pinnatifida, HFD containing Laminaria Japonica (LJ), HFD containing Sargassum Fulvellum, or HFD containing Hizikia Fusiforme (HF) for 16 weeks.
RESULTS
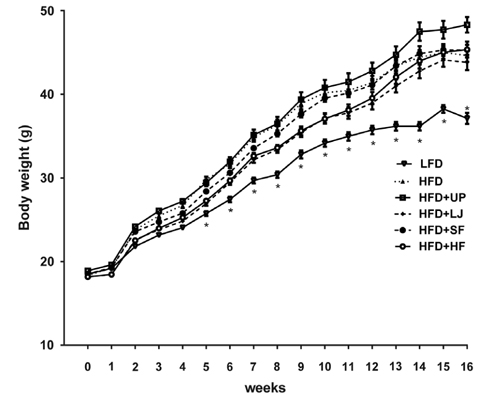
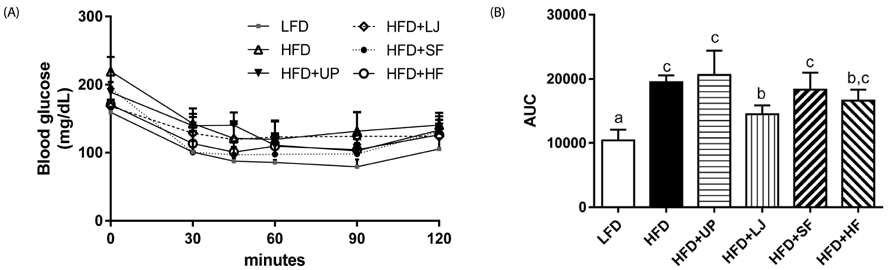
Brown seaweed supplementation did not affect long-term HFD-associated changes in body weight or adiposity, although mice fed HFD + LJ or HFD + HF gained slightly less body weight compared with those fed HFD at the beginning of feeding. Despite being obese, mice fed HFD + LJ appeared to show improved insulin sensitivity compared to mice fed HFD. Consistently, we observed significantly reduced blood glucose concentrations in mice fed HFD + LJ compared with those of mice fed HFD. Although no significant differences in adipocyte size were detected among the HFD-fed groups, consumption of seaweeds decreased formation of HFD-induced crown-like structures in gonadal adipose tissue as well as plasma inflammatory cytokines. BMDM from mice fed HFDs with seaweeds showed differential regulation of pro-inflammatory cytokines such as IL-1beta and IL-6 compared with BMDM from mice fed HFD by LPS stimulation.
CONCLUSION
Although seaweed consumption did not prevent long-term HFD-induced obesity in C57BL/6N mice, it reduced insulin resistance (IR) and circulation of pro-inflammatory cytokines. Therefore, seaweeds may ameliorate systemic inflammation and IR in obesity partially due to inhibition of inflammatory signaling in adipose tissue cells as well as bone marrow-derived immune cells.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Hypotriglyceridemic effects of brown seaweed consumption via regulation of bile acid excretion and hepatic lipogenesis in high fat diet-induced obese mice
A-Reum Han, Jae-Hoon Kim, Eunyoung Kim, Jiamei Cui, In-Suk Chai, Guiguo Zhang, Yunkyoung Lee
Nutr Res Pract. 2020;14(6):580-592. doi: 10.4162/nrp.2020.14.6.580.
Reference
-
1. Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996; 87:377–389.
Article2. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995; 95:2409–2415.
Article3. Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001; 280:E827–E847.
Article4. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005; 46:2347–2355.
Article5. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007; 132:2169–2180.
Article6. Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008; 49:1562–1568.
Article7. Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007; 56:2910–2918.
Article8. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830.
Article9. Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski A. Genistein as an anti-inflammatory agent. Inflamm Res. 2003; 52:341–346.
Article10. Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007; 2007:45673.11. Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001; 56:683–687.
Article12. Nam YR, Won SB, Chung YS, Kwak CS, Kwon YH. Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet. Nutr Res Pract. 2015; 9:235–241.
Article13. Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Disord Drug Targets. 2007; 7:135–144.
Article14. Ban JO, Oh JH, Kim TM, Kim DJ, Jeong HS, Han SB, Hong JT. Anti-inflammatory and arthritic effects of thiacremonone, a novel sulfur compound isolated from garlic via inhibition of NF-kappaB. Arthritis Res Ther. 2009; 11:R145.15. Moloney F, Toomey S, Noone E, Nugent A, Allan B, Loscher CE, Roche HM. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes. 2007; 56:574–582.
Article16. MacArtain P, Gill CI, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutr Rev. 2007; 65:535–543.
Article17. Han YR, Ali M, Woo M, Jung HA, Choi JS. Anti-diabetic and anti-inflammatory potential of the edible brown alga hizikia fusiformis. J Food Biochem. 2015; 39:417–428.
Article18. Nwosu F, Morris J, Lund VA, Stewart D, Ross HA, McDougall GJ. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011; 126:1006–1012.
Article19. Kang M, Wijesinghe W, Lee S, Kang S, Ko S, Yang X, Kang N, Jeon B, Kim J, Lee D, Jeon Y. Dieckol isolated from brown seaweed ecklonia cava attenuates type II diabetes in db/db mouse model. Food Chem Toxicol. 2013; 53:294–298.
Article20. Kang SI, Kim MH, Shin HS, Kim HM, Hong YS, Park JG, KO HC, Lee NH, Chung WS, Kim SJ. A water-soluble extract of Petalonia binghamiae inhibits the expression of adipogenic regulators in 3T3-L1 preadipocytes and reduces adiposity and weight gain in rats fed a high-fat diet. J Nutr Biochem. 2010; 21:1251–1257.
Article21. Kang S, Heo S, Kim K, Lee S, Jeon Y. Isolation and identification of new compound, 2, 7 ''-phloroglucinol-6, 6'-bieckol from brown algae, ecklonia cava and its antioxidant effect. J Funct Foods. 2012; 4:158–166.
Article22. DeFuria J, Bennett G, Strissel KJ, Perfield JW 2nd, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr. 2009; 139:1510–1516.
Article23. Perfield JW 2nd, Lee Y, Shulman GI, Samuel VT, Jurczak MJ, Chang E, Xie C, Tsichlis PN, Obin MS, Greenberg AS. Tumor progression locus 2 (TPL2) regulates obesity-associated inflammation and insulin resistance. Diabetes. 2011; 60:1168–1176.
Article24. Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): Isolation and applications. CSH Protoc. 2008; 2008:pdb.prot5080.
Article25. Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010; 59:105–109.
Article26. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808.
Article27. Lesniewski LA, Hosch SE, Neels JG, de Luca C, Pashmforoush M, Lumeng CN, Chiang SH, Scadeng M, Saltiel AR, Olefsky JM. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat Med. 2007; 13:455–462.
Article28. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006; 116:3015–3025.
Article29. World Health Organization (CH). Obesity: Preventing and Managing the Global Epidemic. In : WHO Consultation on Obesity; 3-5 June 1997; Geneva. Geneva: World Health Organization;1998.30. Kopelman PG. Obesity as a medical problem. Nature. 2000; 404:635–643.
Article31. Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010; 314:1–16.
Article32. Li X, Yu Z, Long S, Guo Y, Duan D. Hypoglycemic effect of laminaria japonica polysaccharide in a type 2 diabetes mellitus mouse model. ISRN Endocrinol. 2012; 2012:507462.
Article33. Jang WS, Choung SY. Antiobesity effects of the ethanol extract of laminaria japonica areshoung in high-fat-diet-induced obese rat. Evid Based Complement Alternat Med. 2013; 2013:492807.34. Kang SI, Jin YJ, Ko HC, Choi SY, Hwang JH, Whang I, Kim MH, Shin HS, Jeong HB, Kim SJ. Petalonia improves glucose homeostasis in streptozotocin-induced diabetic mice. Biochem Biophys Res Commun. 2008; 373:265–269.
Article35. Park HJ, Lee MK, Park YB, Shin YC, Choi MS. Beneficial effects of undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem Toxicol. 2011; 49:727–733.
Article36. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007; 356:1517–1526.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of resveratrol on the insulin signaling pathway of obese mice
- The Effects of the Sasa Borealis Leaves Extract on Plasma Adiponectin, Resistin, C-Reactive Protein and Homocysteine Levels in High Fat Diet-Induced Obese C57/BL6J Mice
- Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet
- Effects of caloric restriction on the expression of lipocalin-2 and its receptor in the brown adipose tissue of high-fat diet-fed mice
- Capsanthin Inhibits both Adipogenesis in 3T3-L1 Preadipocytes and Weight Gain in High-Fat Diet-Induced Obese Mice