J Korean Ophthalmol Soc.
2008 Dec;49(12):1989-1995.
Role of Ascorbic Acid Against the Oxidative Stress in Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu College of Medicine, Daegu, Korea. jwkim@cu.ac.kr
Abstract
-
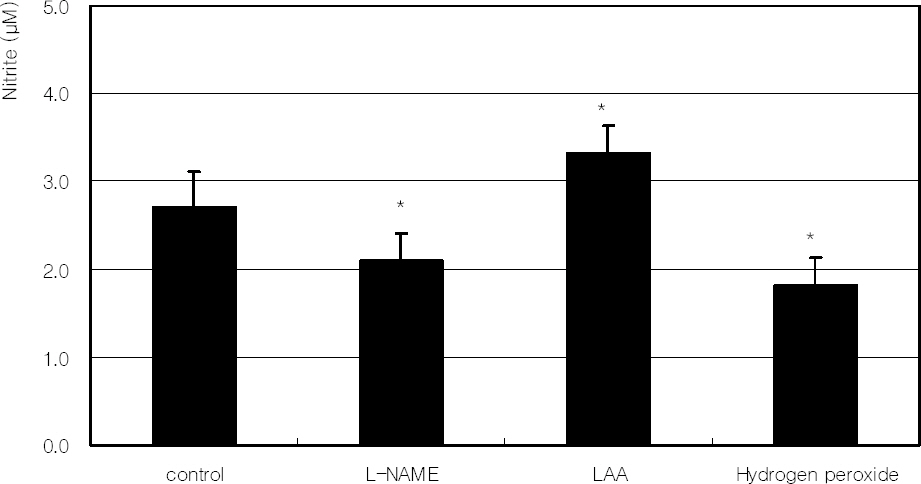
PURPOSE: To investigate the role of L-ascorbic acid (LAA) on the survival and its association with nitric oxide (NO) production against hydrogen peroxide-induced oxidative stress in trabecular meshwork (TM) cells.
METHODS
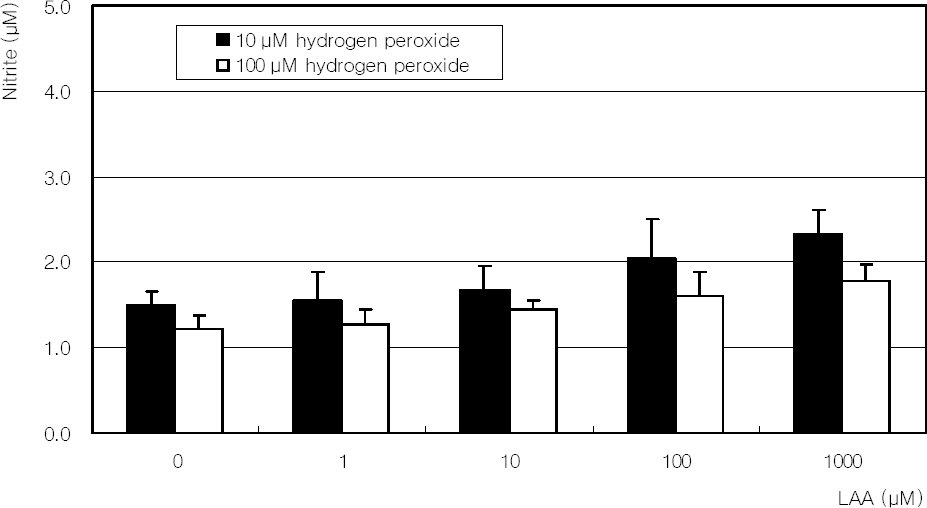
Primarily cultured human TM cells were exposed to hydrogen peroxide; 10, 100 micrometer for 2 days, or 1 mM single exposure with or without co-exposure of LAA. Cellular survival and nitrite production were assessed with MTT and Griess assays, respectively. Flow cytometry using annexin/PI double staining was performed to evaluate apoptosis.
RESULTS
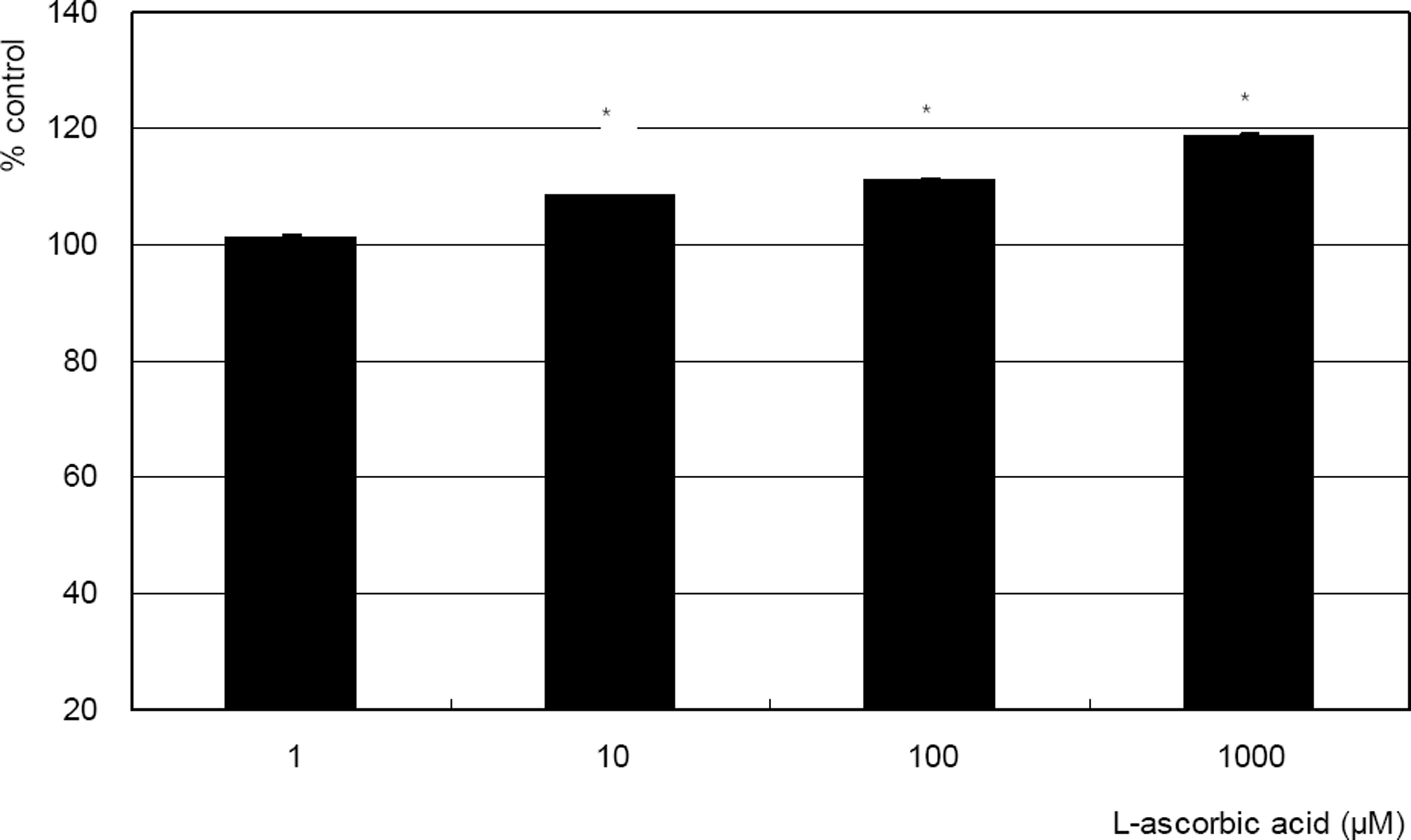
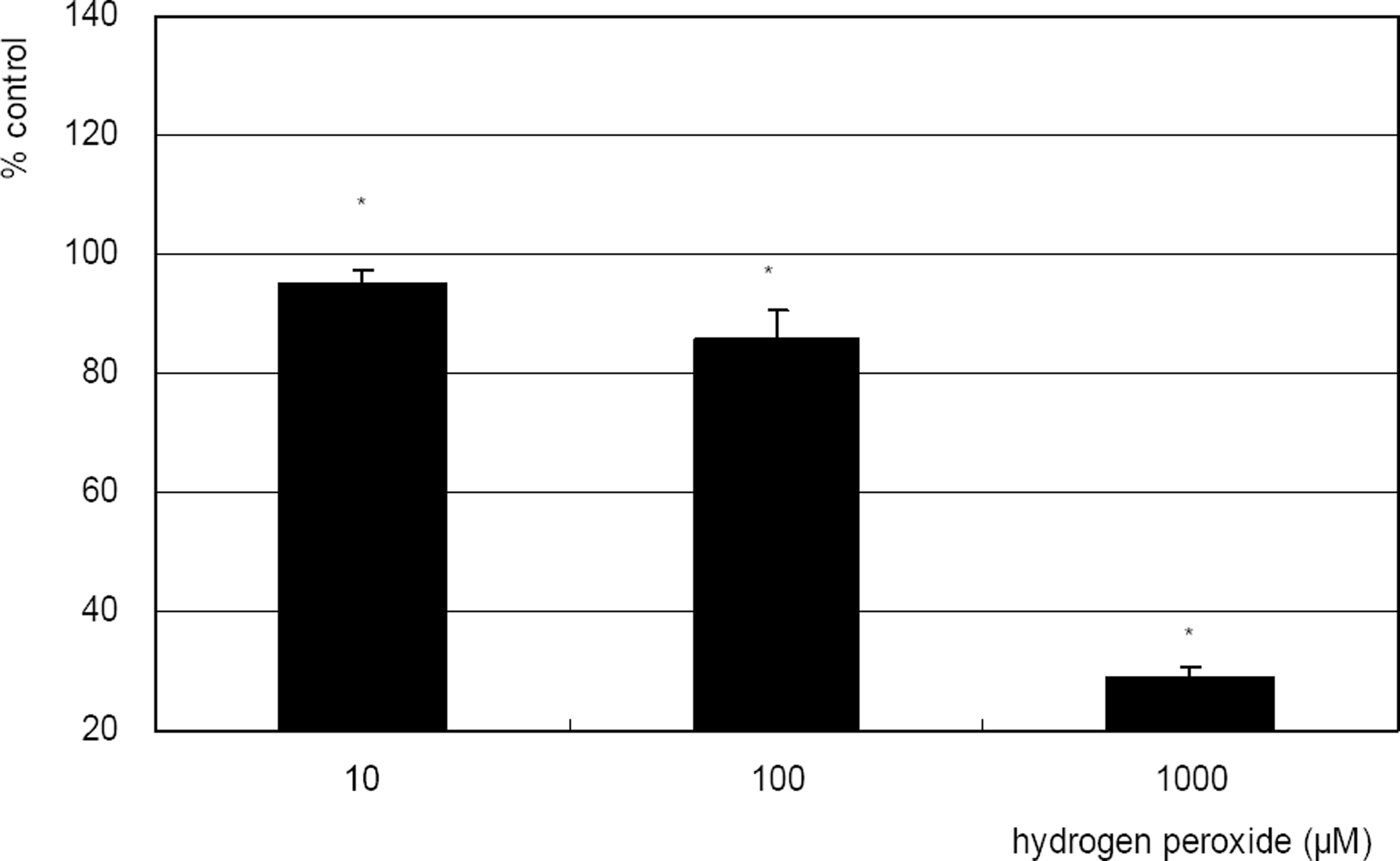
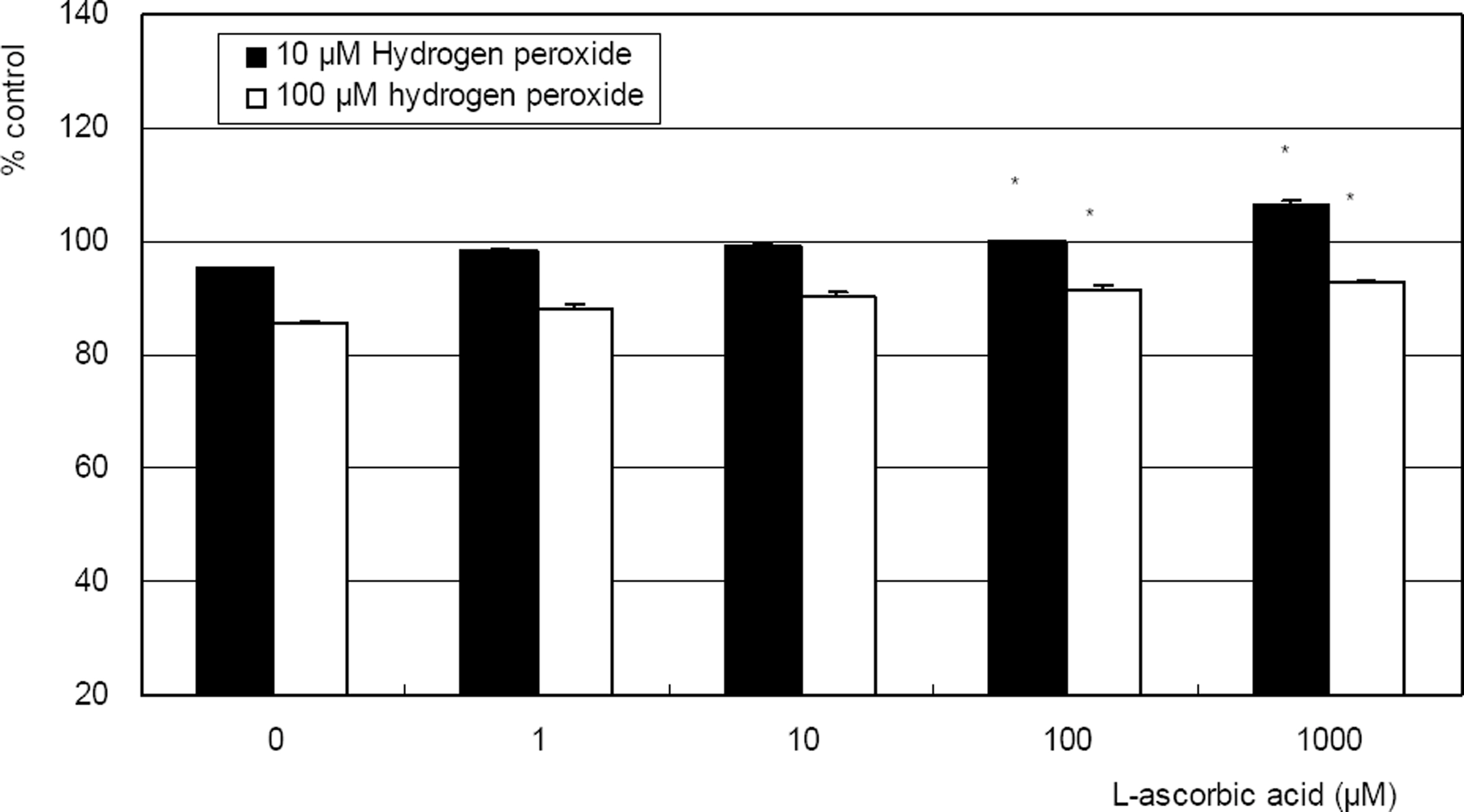
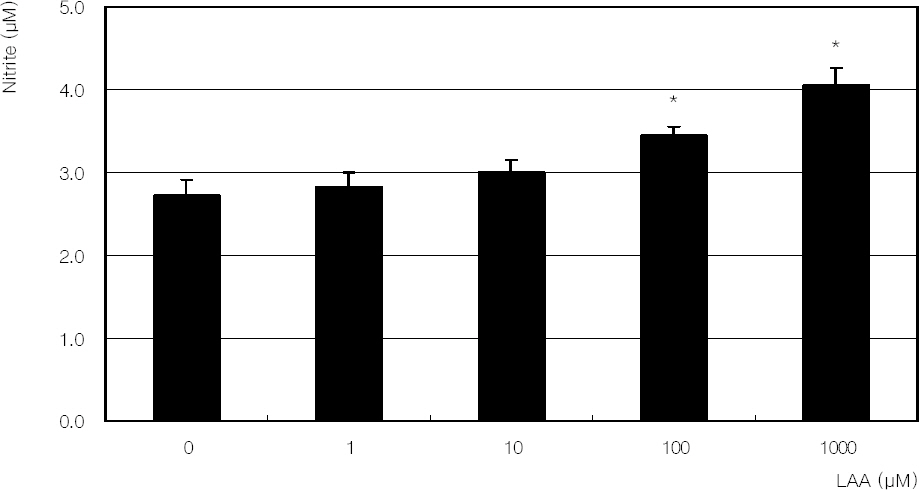
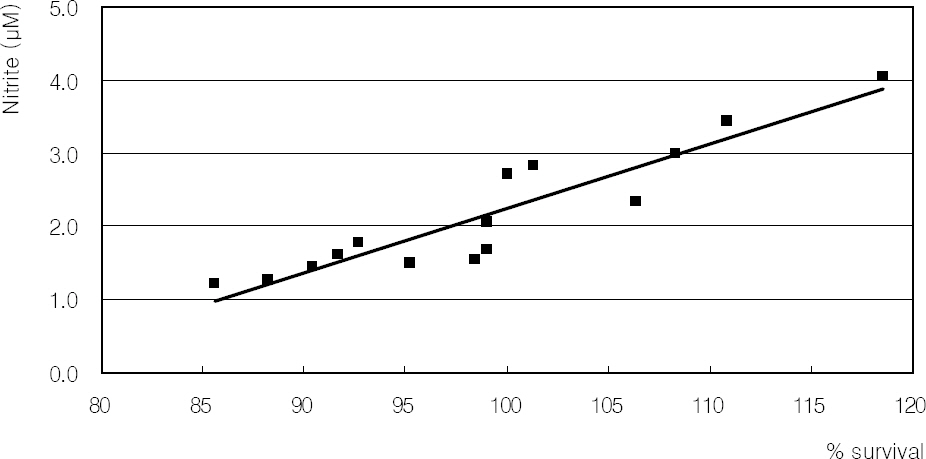
Hydrogen peroxide decreased cellular survival significantly in a dose-dependent manner accompanied with decreased NO production. However cellular survival and NO production were increased significantly with co-exposure of LAA. Cellular survival and NO were highly correlated. Flow cytometric analysis revealed that LAA inhibited hydrogen peroxide-induced apoptosis.
CONCLUSIONS
LAA demonstrated a cytoprotective effect against the hydrogen peroxide-induced oxidative stress accompanied with increased NO production. The cytoprotective effect of LAA may be mediated by preserving NO in TM cells.
MeSH Terms
Figure
Reference
-
References
1. Becker B. Chemical composition of human aqueous humor. Effects of acetazolamide. AMA Arch Ophthalmol. 1957; 57:793–800.2. Erb C, Nau-Staudt K, Flammer J, Nau W. Ascorbic acid as a free radical scavenger in porcine and bovine aqueous humour. Ophthalmic Res. 2004; 36:38–42.
Article3. Heller R, Münscher-Pailug F, Gräbner R, Till U. L-ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem. 1999; 274:8254–60.
Article4. Kim JW. Ascorbic acid enhances nitric oxide production in cultured trabecular meshwork cell. Korean J Ophthalmol. 2005; 19:227–32.5. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.6. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.
Article7. Brüne B, von Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998; 351:261–72.
Article8. Beck K-F, Eberhardt W, Frank S. . Inducible NO synthase: Role in cell signaling. J Exp Biol. 1999; 202:645–53.9. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.10. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eyes. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.11. Meyer P, Champion C, Schlotzer-Schrehardt U. . Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.
Article12. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and ocular facility in monkeys. Exp Eye Res. 1994; 58:99–105.13. Wana RF, Podos SM. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.14. Nathanson JA, McKee M. Alteration of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.15. Matsuo T. Basal nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–5.
Article16. Green LC, Wagner DA, Glogoski J. . Analysis of nitrate, nitrite and 15N nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.17. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.18. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.19. Ross R. Atherosclerosis-an inflammatory disease. N Eng J Med. 1999; 340:115–26.20. May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000; 28:1421–9.21. Huang A, Vita JA, Venema RC, Keaney JF Jr. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000; 275:17399–406.
Article22. Heller R, Unbehaun A, Schnellenberg B. . L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001; 276:40–7.
Article23. Padgaonkar V, Giblin FJ, Leverenz V. . Studies of H2O2-induced effects on cultured bovine trabecular meshwork cells. J Glaucoma. 1994; 3:123–31.
Article24. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthase: structure, function, and inhibition. Biochem J. 2001; 357:593–615.25. Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005; 25:29–38.
Article26. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.
Article27. Millard CB, Tripathi BJ, Tripathi RC. Age-related changes in protein profiles of the normal human trabecular meshwork. Exp Eye Res. 1987; 45:623–31.
Article28. Schchschabel DO, Binninger E. Aging of trabecular meshwork cells of the human eye in vitro. Z Gerontol. 1990; 23:133–5.29. Horstmann HJ, Rohen JW, Sames K. Age-related changes in the composition of proteins in the trabecular meshwork of the human eye. Mech Ageing Dev. 1983; 21:121–36.
Article30. Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000; 87:540–2.
Article31. Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from ocular tissue: the trabecular meshwork. J Cell Physiol. 1999; 180:182–9.32. Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004; 117:2417–26.
Article33. von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 2000; 908:99–110.
Article34. Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med. 1998; 217:53–63.
Article35. Furumoto K, Inoue E, Nagao N. . Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998; 63:935–48.
Article36. Roques SC, Landrault N, Teisse’dre P. . Hydrogen peroxide generation in Caco-2 cell culture medium by addition of phenolic compounds: Effect of ascorbic acid. Free Radic Res. 2002; 36:593–9.
Article37. Chen JZ, Kadlubar FF. A new clue to glaucoma pathogenesis. Am J Med. 2003; 114:697–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- Effect of Ascorbic Acid Against the Oxidative Stress-Induced Cellular Senescence in Trabecular Meshwork Cells
- Ascorbic Acid Enhances Nitric Oxide Production in Trabecular Meshwork Cells
- Effect of High Glucose on the Oxidative Stress in Trabecular Meshwork Cells
- Age-related Changes of the Cellularity and Acid Mucopolysaccharides in the Trabecular Meshwork of the Normal Korean