Korean Circ J.
2018 Mar;48(3):198-208. 10.4070/kcj.2017.0200.
Independent Association of Serum Aldosterone Level with Metabolic Syndrome and Insulin Resistance in Korean Adults
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Family Medicine, St. Vincent's Hospital, The Catholic University of Korea College of Medicine, Suwon, Korea.
- 3Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, Kyung Hee University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 5Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
- 6Department of Internal Medicine, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea.
- 7Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. limsoo@snu.ac.kr
- KMID: 2406923
- DOI: http://doi.org/10.4070/kcj.2017.0200
Abstract
- BACKGROUND AND OBJECTIVES
A relationship between renin-angiotensin system (RAS) components and metabolic syndrome (MetS) has been suggested, but not elucidated clearly. We examined the levels of RAS components in patients with and without MetS and their association with MetS in Korean population.
METHODS
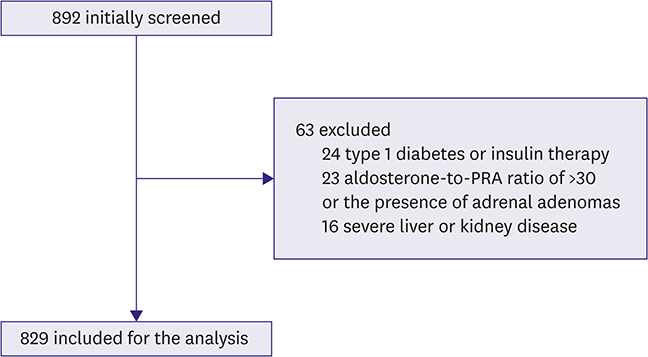
This study was approved by the review boards of the participating institutions and endorsed by the Korean Society of Lipid and Atherosclerosis. We screened 892 Koreans aged ≥20 years who underwent evaluation of hypertension, diabetes, or dyslipidemia at 6 tertiary hospitals in 2015-2016. After excluding patients who were taking diuretics, β-blockers, or RAS blockers, or suspected of primary aldosteronism, 829 individuals were enrolled. Anthropometric and biochemical parameters including aldosterone, plasma renin activity (PRA), and aldosterone-to-PRA ratio were evaluated. The homeostasis model assessment for insulin resistance (HOMA-IR) were used for evaluating insulin resistance.
RESULTS
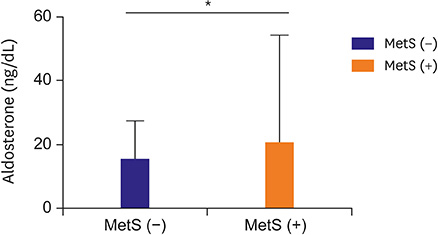
The mean age of the participants was 52.8±12.8 years, 56.3% were male, and their mean systolic and diastolic blood pressures were 133.9±20.0 and 81.2±14.6 mmHg, respectively. The levels of serum aldosterone, but not PRA, were significantly higher in subjects with MetS than in those without (20.6±33.6 vs. 15.3±12.2 ng/dL, p < 0.05), and positively correlated with waist circumference, blood pressure, triglycerides, and glycated hemoglobin. The levels of aldosterone were independently associated with the number of MetS components and HOMA-IR after adjusting for conventional risk factors.
CONCLUSIONS
Serum aldosterone levels were higher in Korean adults with MetS than in those without. This finding suggests that increased aldosterone level might be closely associated with insulin resistance.
MeSH Terms
-
Adult*
Aldosterone*
Atherosclerosis
Blood Pressure
Diuretics
Dyslipidemias
Hemoglobin A, Glycosylated
Homeostasis
Humans
Hyperaldosteronism
Hypertension
Insulin Resistance*
Insulin*
Male
Metabolic Syndrome X
Plasma
Renin
Renin-Angiotensin System
Risk Factors
Tertiary Care Centers
Triglycerides
Waist Circumference
Aldosterone
Diuretics
Insulin
Renin
Triglycerides
Figure
Reference
-
1. Hong AR, Lim S. Clinical characteristics of metabolic syndrome in Korea, and its comparison with other Asian countries. J Diabetes Investig. 2015; 6:508–515.2. Kim CS, Ko SH, Kwon HS, et al. Prevalence, awareness, and management of obesity in Korea: data from the Korea National Health and Nutrition Examination Survey (1998–2011). Diabetes Metab J. 2014; 38:35–43.
Article3. Stiefel P, Vallejo-Vaz AJ, García Morillo S, Villar J. Role of the Renin-Angiotensin system and aldosterone on cardiometabolic syndrome. Int J Hypertens. 2011; 2011:685238.
Article4. Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012; 302:H1219–H1230.
Article5. Pollare T, Lithell H, Berne C. A comparison of the effects of hydrochlorothiazide and captopril on glucose and lipid metabolism in patients with hypertension. N Engl J Med. 1989; 321:868–873.
Article6. Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999; 353:611–616.
Article7. Yusuf S, Gerstein H, Hoogwerf B, et al. Ramipril and the development of diabetes. JAMA. 2001; 286:1882–1885.
Article8. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017; 40:S11–S24.9. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–2572.10. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106:3143–3421.11. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011; 9:48.
Article12. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009; 120:1640–1645.13. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.14. Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85:2402–2410.
Article15. Patel VB, Parajuli N, Oudit GY. Role of angiotensin-converting enzyme 2 (ACE2) in diabetic cardiovascular complications. Clin Sci (Lond). 2014; 126:471–482.
Article16. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993; 329:1456–1462.17. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–869.
Article18. Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004; 351:33–41.
Article19. Rossi GP, Belfiore A, Bernini G, et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008; 93:2566–2571.
Article20. Bentley-Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007; 92:4472–4475.
Article21. Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep. 2011; 13:163–172.
Article22. Jeon JH, Kim KY, Kim JH, et al. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J. 2008; 22:1502–1511.
Article23. Colussi G, Catena C, Lapenna R, Nadalini E, Chiuch A, Sechi LA. Insulin resistance and hyperinsulinemia are related to plasma aldosterone levels in hypertensive patients. Diabetes Care. 2007; 30:2349–2354.
Article24. Kidambi S, Kotchen JM, Grim CE, et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension. 2007; 49:704–711.
Article25. Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab. 2010; 95:1986–1990.
Article26. Catena C, Lapenna R, Baroselli S, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006; 91:3457–3463.
Article27. Fallo F, Federspil G, Veglio F, Mulatero P. The metabolic syndrome in primary aldosteronism. Curr Hypertens Rep. 2007; 9:106–111.
Article28. Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension. 1995; 25:30–36.
Article29. Kathiresan S, Larson MG, Benjamin EJ, et al. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am J Hypertens. 2005; 18:657–665.
Article30. Ingelsson E, Pencina MJ, Tofler GH, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007; 116:984–992.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emerging Role of Aldosterone in Mediating the Vicious Cycle of Obesity, Insulin Resistance and Metabolic Syndrome
- Metabolic Syndrome and Dementia
- Epidemiologic Association between Obesity and Thyroid Nodules
- Correlation of the Serum Testosterone Level with Insulin Resistance and Metabolic Syndrome in Patients of Erectile Dysfunction and Benign Prostatic Hyperplasia
- Interrelationship of Uric Acid, Gout, and Metabolic Syndrome: Focus on Hypertension, Cardiovascular Disease, and Insulin Resistance