Korean J Physiol Pharmacol.
2016 Jul;20(4):415-424. 10.4196/kjpp.2016.20.4.415.
Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages
- Affiliations
-
- 1Department of Microbiology, Gachon University School of Medicine, Incheon 21936, Korea.
- 2Department of Pharmacology, Gachon University School of Medicine, Incheon 21936, Korea. hgcheon@gachon.ac.kr
- 3Gachon Medical Research Institute, Gil Medical Center, Incheon 21565, Korea.
- KMID: 2371059
- DOI: http://doi.org/10.4196/kjpp.2016.20.4.415
Abstract
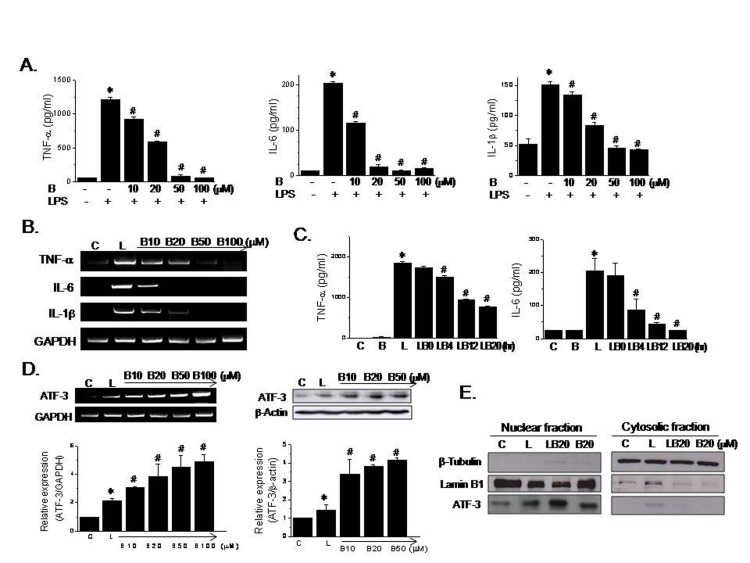
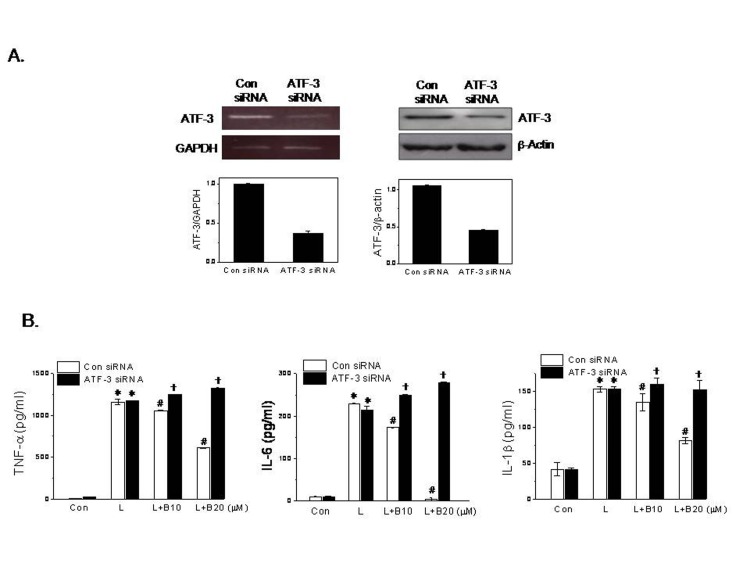
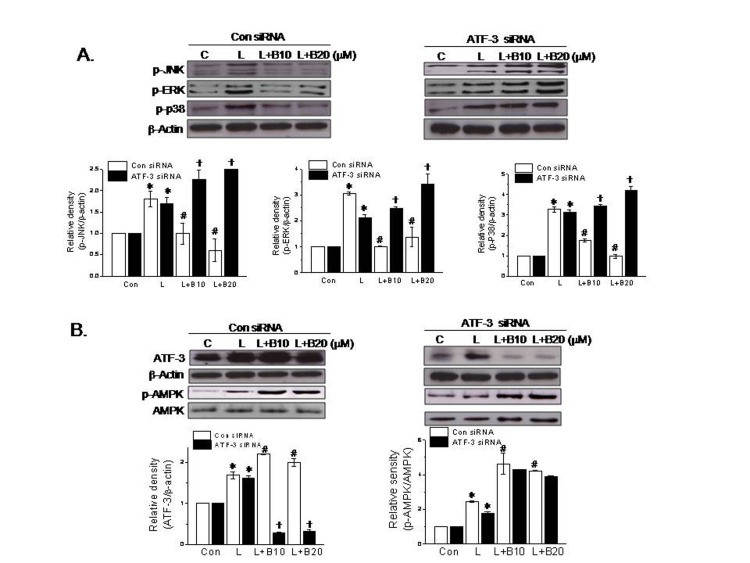
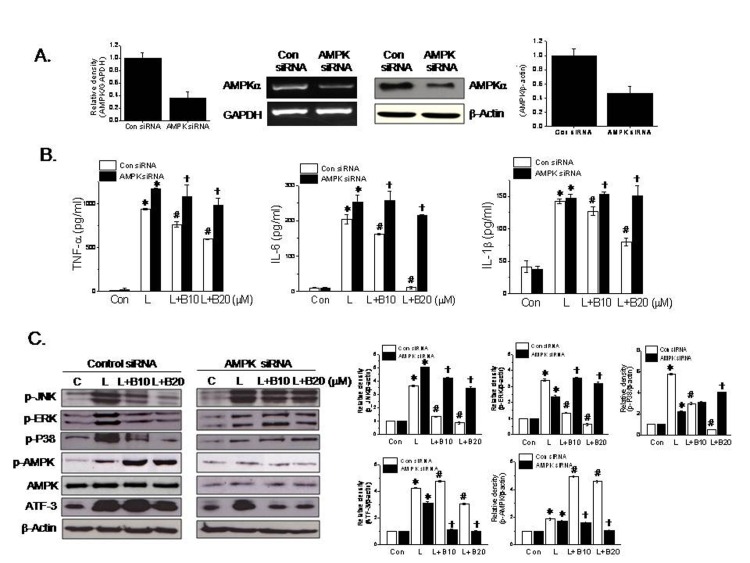
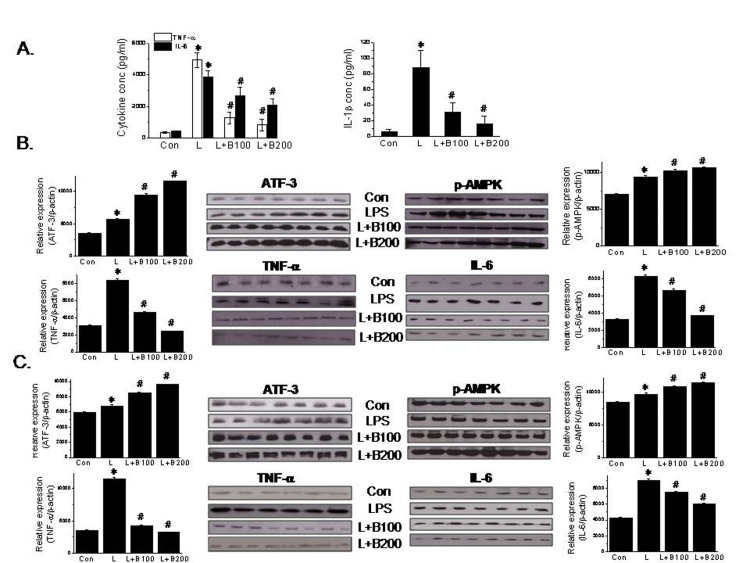
- Berberine is an isoquinoline alkaloid found in Rhizoma coptidis, and elicits anti-inflammatory effects through diverse mechanisms. Based on previous reports that activating transcription factor-3 (ATF-3) acts as a negative regulator of LPS signaling, the authors investigated the possible involvement of ATF-3 in the anti-inflammatory effects of berberine. It was found berberine concentration-dependently induced the expressions of ATF-3 at the mRNA and protein levels and concomitantly suppressed the LPS-induced productions of proinflammatory cytokines (TNF-α, IL-6, and IL-1β). In addition, ATF-3 knockdown abolished the inhibitory effects of berberine on LPS-induced proinflammatory cytokine production, and prevented the berberine-induced suppression of MAPK phosphorylation, but had little effect on AMPK phosphorylation. On the other hand, the effects of berberine, that is, ATF-3 induction, proinflammatory cytokine inhibition, and MAPK inactivation, were prevented by AMPK knockdown, suggesting ATF-3 induction occurs downstream of AMPK activation. The in vivo administration of berberine to mice with LPS-induced endotoxemia increased ATF-3 expression and AMPK phosphorylation in spleen and lung tissues, and concomitantly reduced the plasma and tissue levels of proinflammatory cytokines. These results suggest berberine has an anti-inflammatory effect on macrophages and that this effect is attributable, at least in part, to pathways involving AMPK activation and ATF-3 induction.
MeSH Terms
Figure
Reference
-
1. Schmeller T, Latz-Brüning B, Wink M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry. 1997; 44:257–266. PMID: 9004542.
Article2. Kuo CL, Chou CC, Yung BY. Berberine complexes with DNA in the berberine-induced apoptosis in human leukemic HL-60 cells. Cancer Lett. 1995; 93:193–200. PMID: 7621428.
Article3. Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004; 10:1344–1351. PMID: 15531889.
Article4. Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006; 55:2256–2264. PMID: 16873688.
Article5. Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, Do MS. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med. 2006; 38:599–605. PMID: 17202835.
Article6. Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009; 296:E955–E964. PMID: 19208854.
Article7. Kim KW, Ha KT, Park CS, Jin UH, Chang HW, Lee IS, Kim CH. Polygonum cuspidatum, compared with baicalin and berberine, inhibits inducible nitric oxide synthase and cyclooxygenase-2 gene expressions in RAW 264.7 macrophages. Vascul Pharmacol. 2007; 47:99–107. PMID: 17553752.
Article8. Lee CH, Chen JC, Hsiang CY, Wu SL, Wu HC, Ho TY. Berberine suppresses inf lammatory agents-induced interleukin-1beta and tumor necrosis factor-alpha productions via the inhibition of IkappaB degradation in human lung cells. Pharmacol Res. 2007; 56:193–201. PMID: 17681786.9. Lu DY, Tang CH, Chen YH, Wei IH. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem. 2010; 110:697–705. PMID: 20512929.
Article10. Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004; 203:127–137. PMID: 14732220.
Article11. Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998; 67:821–855. PMID: 9759505.
Article12. Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004; 279:47898–47905. PMID: 15371448.
Article13. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003; 144:5179–5183. PMID: 12960015.
Article14. Cai Y, Zhang C, Nawa T, Aso T, Tanaka M, Oshiro S, Ichijo H, Kitajima S. Homocysteine-responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH(2)-terminal kinase and promoter response element. Blood. 2000; 96:2140–2148. PMID: 10979959.
Article15. Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999; 7:321–335. PMID: 10440233.16. Hashimoto Y, Zhang C, Kawauchi J, Imoto I, Adachi MT, Inazawa J, Amagasa T, Hai T, Kitajima S. An alternatively spliced isoform of transcriptional repressor ATF3 and its induction by stress stimuli. Nucleic Acids Res. 2002; 30:2398–2406. PMID: 12034827.
Article17. Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001; 2:599–609. PMID: 11483993.
Article18. Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, Kennedy K, Hai T, Bolouri H, Aderem A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006; 441:173–178. PMID: 16688168.
Article19. Suganami T, Yuan X, Shimoda Y, Uchio-Yamada K, Nakagawa N, Shirakawa I, Usami T, Tsukahara T, Nakayama K, Miyamoto Y, Yasuda K, Matsuda J, Kamei Y, Kitajima S, Ogawa Y. Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated Fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ Res. 2009; 105:25–32. PMID: 19478204.
Article20. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912. PMID: 12471242.
Article21. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830. PMID: 14679177.
Article22. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808. PMID: 14679176.
Article23. Burcelin R. Regulation of metabolism: a cross talk between gut microbiota and its human host. Physiology (Bethesda). 2012; 27:300–307. PMID: 23026753.
Article24. Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006; 1084:89–117. PMID: 17151295.
Article25. Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009; 58:2574–2582. PMID: 19690061.
Article26. Kaneto H, Kawamori D, Nakatani Y, Gorogawa S, Matsuoka TA. Oxidative stress and the JNK pathway as a potential therapeutic target for diabetes. Drug News Perspect. 2004; 17:447–453. PMID: 15514704.
Article27. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108:1167–1174. PMID: 11602624.
Article28. El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000; 275:223–228. PMID: 10617608.
Article29. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000; 348:607–614. PMID: 10839993.
Article30. Huang NL, Chiang SH, Hsueh CH, Liang YJ, Chen YJ, Lai LP. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol. 2009; 134:169–175. PMID: 18597869.31. Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004; 279:20767–20774. PMID: 14985344.32. Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, Cheon HG. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem. 2014; 289:23246–23255. PMID: 24973221.
Article33. Nilsson R, Bajic VB, Suzuki H, di Bernardo D, Björkegren J, Katayama S, Reid JF, Sweet MJ, Gariboldi M, Carninci P, Hayashizaki Y, Hume DA, Tegner J, Ravasi T. Transcriptional network dynamics in macrophage activation. Genomics. 2006; 88:133–142. PMID: 16698233.
Article34. Ho HH, Antoniv TT, Ji JD, Ivashkiv LB. Lipopolysaccharide-induced expression of matrix metalloproteinases in human monocytes is suppressed by IFN-gamma via superinduction of ATF-3 and suppression of AP-1. J Immunol. 2008; 181:5089–5097. PMID: 18802113.35. Stearns ME, Kim G, Garcia F, Wang M. Interleukin-10 induced activating transcription factor 3 transcriptional suppression of matrix metalloproteinase-2 gene expression in human prostate CPTX-1532 Cells. Mol Cancer Res. 2004; 2:403–416. PMID: 15280448.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling
- Inhibitory Effects of Paeonia Suffruticosa on the Expression of Inducible Nitric Oxide Synthase Gene in RAW264.7 Macrophages
- Anti-Inflammatory Effect of Mangostenone F in Lipopolysaccharide-Stimulated RAW264.7 Macrophages by Suppressing NF-kappaB and MAPK Activation
- Anti-inflammatory and immune enhancing activities of PB203 in mouse macrophage RAW264.7 cells
- JS-III-49, a hydroquinone derivative, exerts anti-inflammatory activity by targeting Akt and p38