Korean J Physiol Pharmacol.
2017 May;21(3):345-352. 10.4196/kjpp.2017.21.3.345.
JS-III-49, a hydroquinone derivative, exerts anti-inflammatory activity by targeting Akt and p38
- Affiliations
-
- 1Department of Genetic Engineering, Sungkyunkwan University, Suwon 16419, Korea. jaecho@skku.edu
- 2Department of Pharmaceutical Engineering, Cheongju University, Cheongju 28503, Korea.
- 3School of Systems Biomedical Science, Soongsil University, Seoul 06978, Korea. kimmy@ssu.ac.kr
- KMID: 2376963
- DOI: http://doi.org/10.4196/kjpp.2017.21.3.345
Abstract
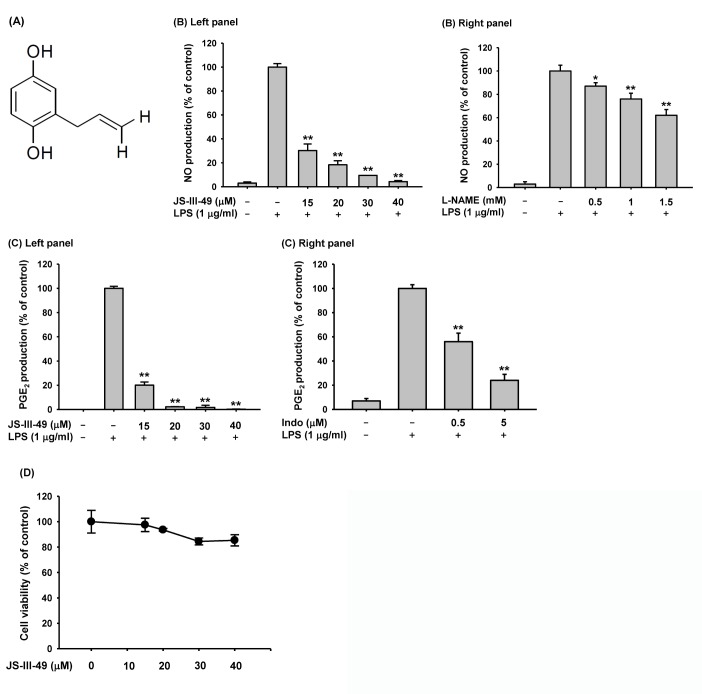
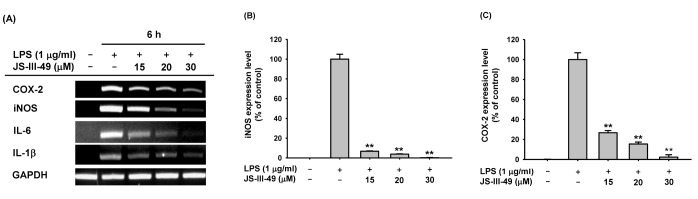
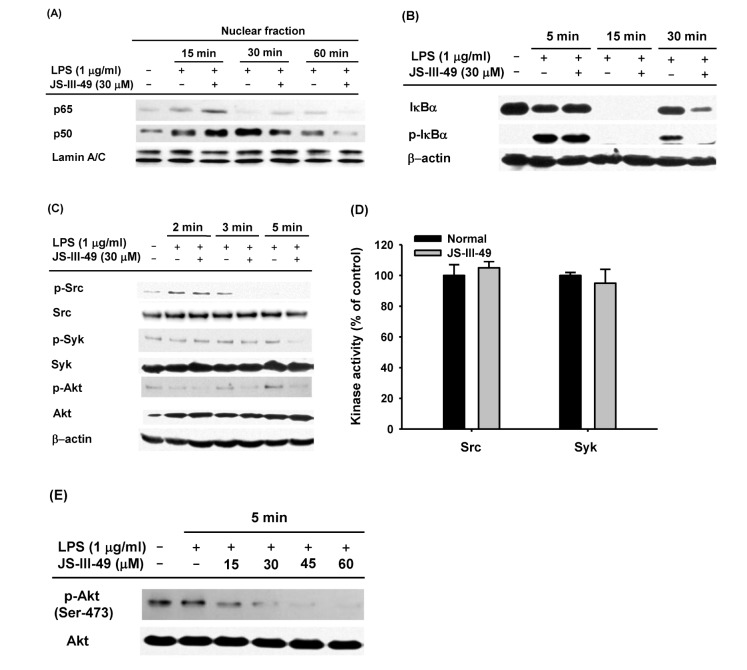
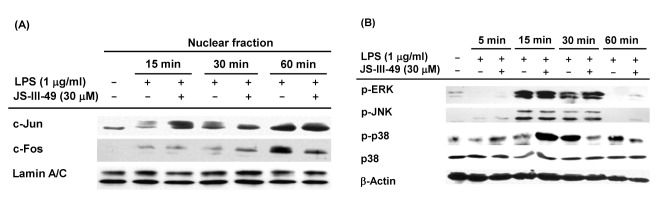
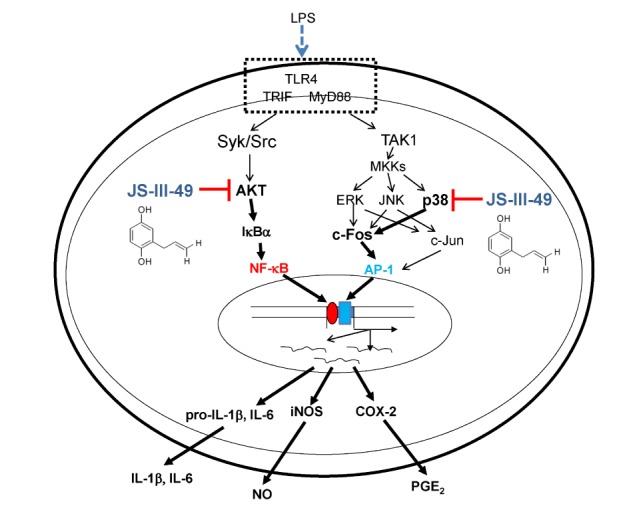
- Since previous studies have reported that hydroquinone (HQ) exerted immunosuppressive and anti-inflammatory activity, various HQ derivatives have been synthesized and their biological activities investigated. In this study, we explored the anti-inflammatory activity of JS-III-49, a novel HQ derivative, in macrophage-mediated inflammatory responses. JS-III-49 suppressed the production of the inflammatory mediators nitric oxide (NO) and prostaglandin E2 (PGE2) and down-regulated the mRNA expression of the inflammatory enzymes cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) as well as the expression of the pro-inflammatory cytokines interleukin-6 (IL-6) and IL-1b without cytotoxicity in LPS-stimulated RAW264.7 cells. JS-III-49 inhibited nuclear translocation of the NF-kB transcription factors p65 and p50 by directly targeting Akt, an upstream kinase of the NF-kB pathway, in LPS-stimulated RAW264.7 cells. However, JS-III-49 did not directly inhibit the kinase activities of Src and Syk, which are upstream kinases of Akt, in LPS-stimulated RAW264.7 cells. Moreover, JS-III-49 suppressed the nuclear translocation of c-Fos, one of the components of AP-1, by specifically targeting p38, an upstream mitogen-activated protein kinase (MAPK) in the AP-1 pathway in LPS-stimulated RAW264.7 cells. These results suggest that JS-III-49 plays an anti-inflammatory role in LPS-stimulated macrophages by targeting Akt and p38 in the NF-kB and AP-1 pathways, respectively.
Keyword
MeSH Terms
-
Cyclooxygenase 2
Cytokines
Dinoprostone
Interleukin-6
Macrophages
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphotransferases
Protein Kinases
RNA, Messenger
Transcription Factor AP-1
Transcription Factors
Cyclooxygenase 2
Cytokines
Dinoprostone
Interleukin-6
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphotransferases
Protein Kinases
RNA, Messenger
Transcription Factor AP-1
Transcription Factors
Figure
Reference
-
1. Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007; 147:227–235. PMID: 17223962.2. Kaur M, Singh M, Silakari O. Inhibitors of switch kinase spleen tyrosine kinase' in inflammation and immune-mediated disorders: a review. Eur J Med Chem. 2013; 67:434–446. PMID: 23917087.
Article3. Massarotti EM. Clinical and patient-reported outcomes in clinical trials of abatacept in the treatment of rheumatoid arthritis. Clin Ther. 2008; 30:429–442. PMID: 18405783.
Article4. Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005; 23:901–944. PMID: 15771589.
Article5. Shao HJ, Lou Z, Jeong JB, Kim KJ, Lee J, Lee SH. Tolfenamic acid suppresses inflammatory stimuli-mediated activation of NF-κB signaling. Biomol Ther (Seoul). 2015; 23:39–44. PMID: 25593642.
Article6. Byeon SE, Yi YS, Oh J, Yoo BC, Hong S, Cho JY. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012; DOI: 10.1155/2012/512926.
Article7. Yi YS, Son YJ, Ryou C, Sung GH, Kim JH, Cho JY. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014; DOI: 10.1155/2014/270302.
Article8. Yu T, Yi YS, Yang Y, Oh J, Jeong D, Cho JY. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012; DOI: 10.1155/2012/979105.
Article9. Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung GH, Yoo BC, Cho JY. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014; DOI: 10.1155/2014/352371.
Article10. Baek KS, Hong YD, Kim Y, Sung NY, Yang S, Lee KM, Park JY, Park JS, Rho HS, Shin SS, Cho JY. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res. 2015; 39:155–161. PMID: 26045689.
Article11. Sung NY, Kim MY, Cho JY. Scutellarein reduces inflammatory responses by inhibiting Src kinase activity. Korean J Physiol Pharmacol. 2015; 19:441–449. PMID: 26330757.
Article12. Kim JH, Kim MY, Kim JH, Cho JY. Fisetin suppresses macrophage-mediated inflammatory responses by blockade of Src and Syk. Biomol Ther (Seoul). 2015; 23:414–420. PMID: 26336580.
Article13. Yang Y, Lee J, Rhee MH, Yu T, Baek KS, Sung NY, Kim Y, Yoon K, Kim JH, Kwak YS, Hong S, Kim JH, Cho JY. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015; 39:61–68. PMID: 25535478.
Article14. Kim Y, Jeong EJ, Han Lee IS, Kim MY, Cho JY. (E)-3-(3-methoxyphenyl)-1-(2-pyrrolyl)-2-propenone displays suppression of inflammatory responses via inhibition of Src, Syk, and NF-κB. Korean J Physiol Pharmacol. 2016; 20:91–99. PMID: 26807028.
Article15. Seo WY, Youn GS, Choi SY, Park J. Butein, a tetrahydroxychalcone, suppresses pro-inflammatory responses in HaCaT keratinocytes. BMB Rep. 2015; 48:495–500. PMID: 25541056.
Article16. DeCaprio AP. The toxicology of hydroquinone--relevance to occupational and environmental exposure. Crit Rev Toxicol. 1999; 29:283–330. PMID: 10379810.17. McDonald TA, Holland NT, Skibola C, Duramad P, Smith MT. Hypothesis: phenol and hydroquinone derived mainly from diet and gastrointestinal flora activity are causal factors in leukemia. Leukemia. 2001; 15:10–20. PMID: 11243376.
Article18. Kim AR, Cho JY, Lee JY, Choi JS, Chung HY. Hydroquinone modulates reactivity of peroxynitrite and nitric oxide production. J Pharm Pharmacol. 2005; 57:475–481. PMID: 15831208.
Article19. Pyatt DW, Yang Y, Stillman WS, Cano LL, Irons RD. Hydroquinone inhibits PMA-induced activation of NFkappaB in primary human CD19+ B lymphocytes. Cell Biol Toxicol. 2000; 16:41–51. PMID: 10890505.20. Lee JY, Lee YG, Lee J, Yang KJ, Kim AR, Kim JY, Won MH, Park J, Yoo BC, Kim S, Cho WJ, Cho JY. Akt Cys-310-targeted inhibition by hydroxylated benzene derivatives is tightly linked to their immuno-suppressive effects. J Biol Chem. 2010; 285:9932–9948. PMID: 20054000.
Article21. Ma Q, Kinneer K, Ye J, Chen BJ. Inhibition of nuclear factor kappaB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Mol Pharmacol. 2003; 64:211–219. PMID: 12869625.22. Lee JY, Kim JY, Lee YG, Shin WC, Chun T, Rhee MH, Cho JY. Hydroquinone, a reactive metabolite of benzene, reduces macrophage-mediated immune responses. Mol Cells. 2007; 23:198–206. PMID: 17464197.23. Li Q, Geiselhart L, Mittler JN, Mudzinski SP, Lawrence DA, Freed BM. Inhibition of human T lymphoblast proliferation by hydroquinone. Toxicol Appl Pharmacol. 1996; 139:317–323. PMID: 8806848.
Article24. Choi JM, Cho YC, Cho WJ, Kim TS, Kang BY. Hydroquinone, a major component in cigarette smoke, reduces IFN-gamma production in antigen-primed lymphocytes. Arch Pharm Res. 2008; 31:337–341. PMID: 18409047.25. Lee MH, Chung SW, Kang BY, Kim KM, Kim TS. Hydroquinone, a reactive metabolite of benzene, enhances interleukin-4 production in CD4+ T cells and increases immunoglobulin E levels in antigen-primed mice. Immunology. 2002; 106:496–502. PMID: 12153512.
Article26. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138. PMID: 7181105.
Article27. Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000; 398:399–407. PMID: 10862830.
Article28. Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986; 94:57–63. PMID: 3782817.
Article29. Park JG, Son YJ, Aravinthan A, Kim JH, Cho JY. Korean Red Ginseng water extract arrests growth of xenografted lymphoma cells. J Ginseng Res. 2016; 40:431–436. PMID: 27746697.
Article30. Yang WS, Jeong D, Yi YS, Park JG, Seo H, Moh SH, Hong S, Cho JY. IRAK1/4-targeted anti-inflammatory action of caffeic acid. Mediators Inflamm. 2013; DOI: 10.1155/2013/518183.
Article31. Baek KS, Yi YS, Son YJ, Yoo S, Sung NY, Kim Y, Hong S, Aravinthan A, Kim JH, Cho JY. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016; 40:437–444. PMID: 27746698.32. Yu T, Ahn HM, Shen T, Yoon K, Jang HJ, Lee YJ, Yang HM, Kim JH, Kim C, Han MH, Cha SH, Kim TW, Kim SY, Lee J, Cho JY. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011; 137:1197–1206. PMID: 21821108.
Article33. Yu T, Lee J, Lee YG, Byeon SE, Kim MH, Sohn EH, Lee YJ, Lee SG, Cho JY. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer tegmentosum. J Ethnopharmacol. 2010; 128:139–147. PMID: 20045722.
Article34. Jeong HY, Sung GH, Kim JH, Yoon JY, Yang Y, Park JG, Kim SH, Yi YS, Yang WS, Yoon DH, Kim TW, Kim JH, Cho JY. Syk and Src are major pharmacological targets of a Cerbera manghas methanol extract with kaempferol-based anti-inflammatory activity. J Ethnopharmacol. 2014; 151:960–969. PMID: 24342777.
Article35. Choi JY, Kwun MJ, Kim KH, Lyu JH, Han CW, Jeong HS, Ha KT, Jung HJ, Lee BJ, Sadikot RT, Christman JW, Jung SK, Joo M. Protective effect of the fruit hull of Gleditsia sinensis on LPS-induced acute lung injury is associated with Nrf2 activation. Evid Based Complement Alternat Med. 2012; DOI: 10.1155/2012/974713.
Article36. Kim E, Kang BY, Kim TS. Inhibition of interleukin-12 production in mouse macrophages by hydroquinone, a reactive metabolite of benzene, via suppression of nuclear factor-kappaB binding activity. Immunol Lett. 2005; 99:24–29. PMID: 15894107.37. Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000; 58:1026–1034. PMID: 11040050.
Article38. Yang S, Kim Y, Jeong D, Kim JH, Kim S, Son YJ, Yoo BC, Jeong EJ, Kim TW, Lee IH, Cho JY. Pyrrole-derivative of chalcone, (e)-3-phenyl-1-(2-pyrrolyl)-2-propenone, inhibits inflammatory responses via inhibition of Src, Syk, and tak1 kinase activities. Biomol Ther (Seoul). 2016; 24:595–603. PMID: 27469142.
Article39. Hossen MJ, Baek KS, Kim E, Yang WS, Jeong D, Kim JH, Kweon DH, Yoon DH, Kim TW, Kim JH, Cho JY. In vivo and in vitro anti-inflammatory activities of Persicaria chinensis methanolic extract targeting Src/Syk/NF-κB. J Ethnopharmacol. 2015; 159:9–16. PMID: 25446596.
Article40. Yu T, Li YJ, Bian AH, Zuo HB, Zhu TW, Ji SX, Kong F, Yin DQ, Wang CB, Wang ZF, Wang HQ, Yang Y, Yoo BC, Cho JY. The regulatory role of activating transcription factor 2 in inflammation. Mediators Inflamm. 2014; DOI: 10.1155/2014/950472.
Article41. Ha SK, Sung J, Choi I, Kim Y. Oryza sativa (Rice) Hull extract inhibits lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and nuclear factor-κB activation. Pharmacogn Mag. 2016; 12:295–301. PMID: 27867272.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phosphorylation of Akt Mediates Anti-Inflammatory Activity of 1-p-Coumaroyl beta-D-Glucoside Against Lipopolysaccharide-Induced Inflammation in RAW264.7 Cells
- Hydroquinone suppresses IFN-β expression by targeting AKT/IRF3 pathway
- 3,4,5-Trihydroxycinnamic Acid Inhibits Lipopolysaccharide-Induced Inflammatory Response through the Activation of Nrf2 Pathway in BV2 Microglial Cells
- Animal Experiments of Cutaneous Depigmentation by 4-Isoprophlcatechol and Hydroquinone
- N-(p-Coumaryol)-Tryptamine Suppresses the Activation of JNK/c-Jun Signaling Pathway in LPS-Challenged RAW264.7 Cells