Ann Dermatol.
2016 Dec;28(6):740-748. 10.5021/ad.2016.28.6.740.
Pretreatment of Ferulic Acid Protects Human Dermal Fibroblasts against Ultraviolet A Irradiation
- Affiliations
-
- 1Department of Dermatology, Konyang University College of Medicine, Daejeon, Korea.
- 2Department of Biological Engineering, Graduate School of Engineering, Konkuk University, Seoul, Korea. gucci7796@hanmail.net
- 3Department of Dermatology, Konkuk University School of Medicine, Seoul, Korea. kjahn@kuh.ac.kr
- KMID: 2368125
- DOI: http://doi.org/10.5021/ad.2016.28.6.740
Abstract
- BACKGROUND
Approximately 90%~99% of ultraviolet A (UVA) ray reaches the Earth's surface. The deeply penetrating UVA rays induce the formation of reactive oxygen species (ROS), which results in oxidative stress such as photoproducts, senescence, and cell death. Thus, UVA is considered a primary factor that promotes skin aging.
OBJECTIVE
Researchers investigated whether pretreatment with ferulic acid protects human dermal fibroblasts (HDFs) against UVA-induced cell damages.
METHODS
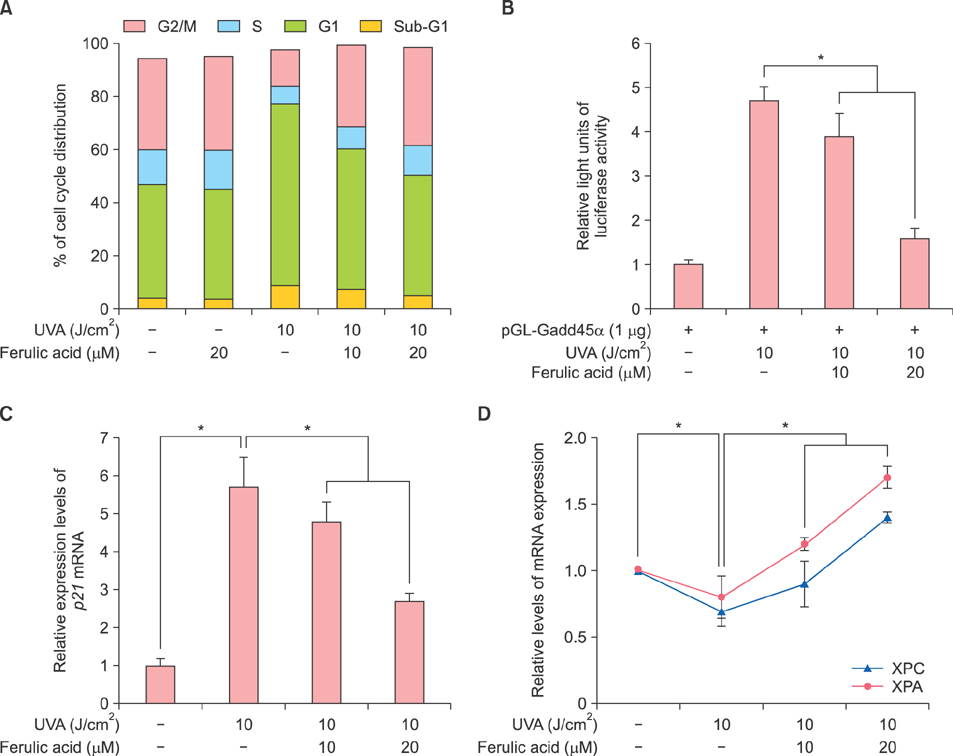
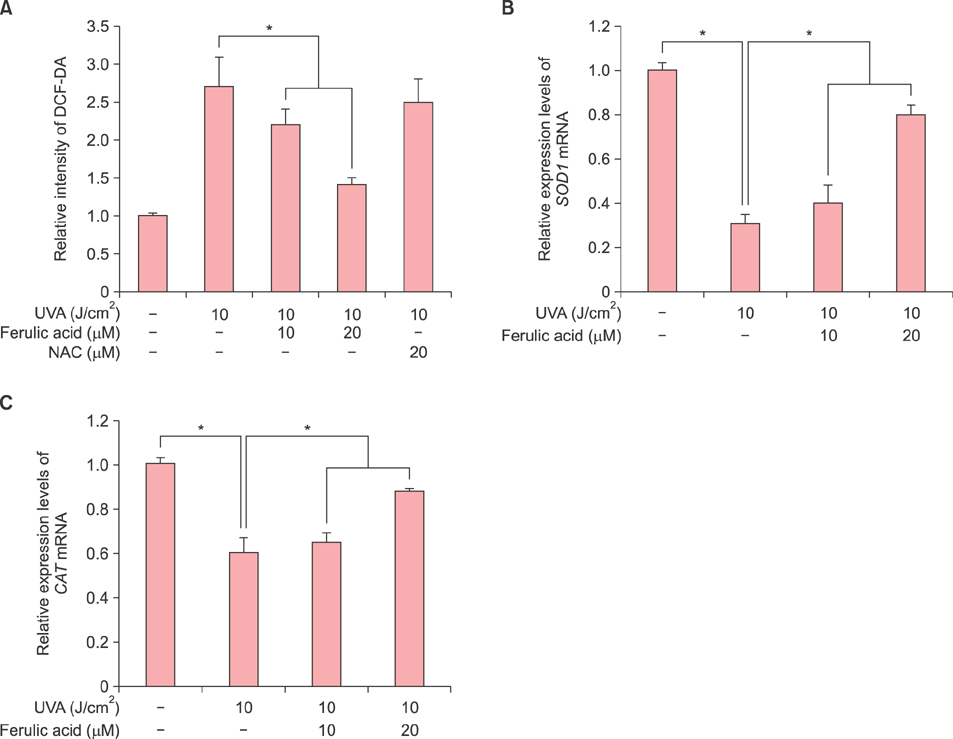
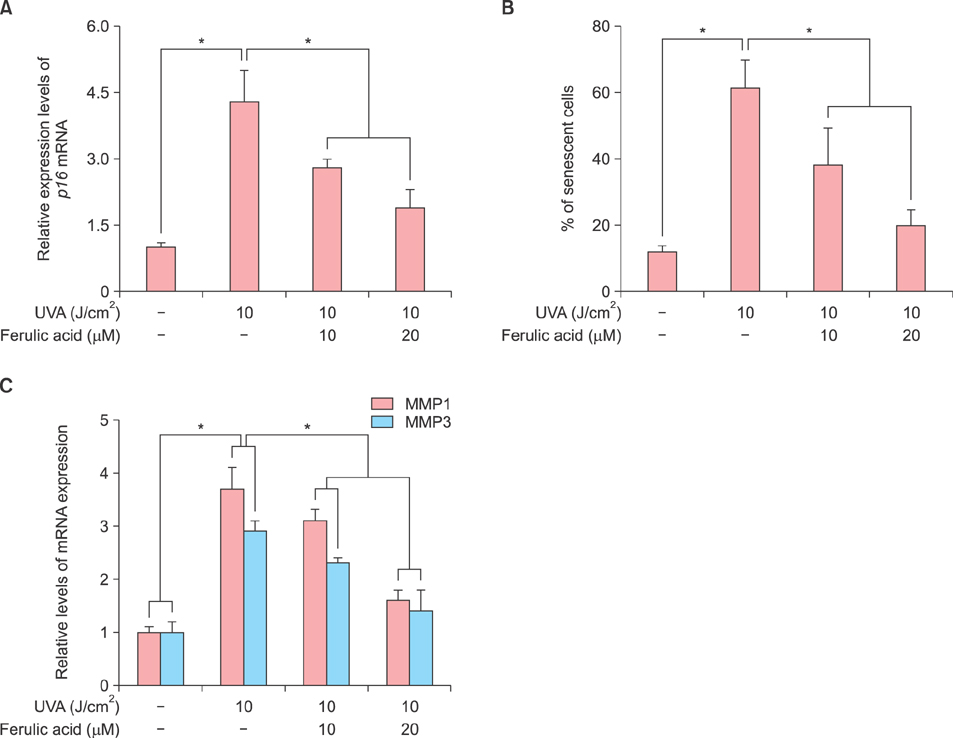
HDF proliferation was analyzed using the water-soluble tetrazolium salt assay. Cell cycle distribution and intracellular ROS levels were assessed by flow cytometric analysis. Senescence was evaluated using a senescence-associated β-galactosidase assay, while Gadd45α promoter activity was analyzed through a luciferase assay. The expression levels of superoxide dismutase 1 (SOD1), catalase (CAT), xeroderma pigmentosum complementation group A and C, matrix metalloproteinase 1 and 3, as well as p21 and p16 were measured using quantitative real-time polymerase chain reaction.
RESULTS
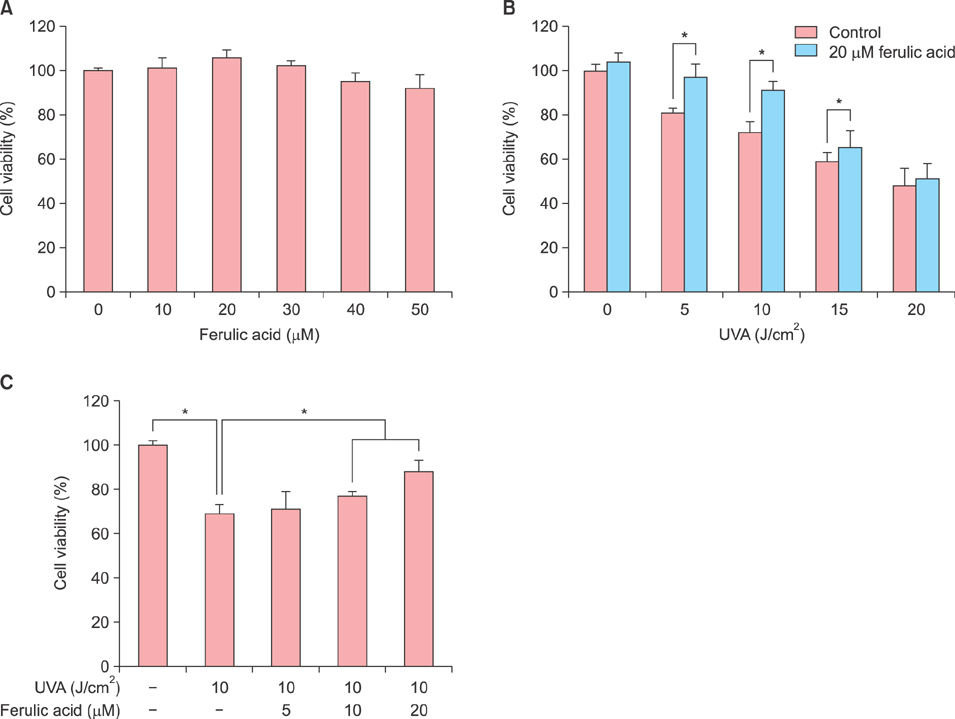
Inhibition of proliferation and cell cycle arrest were detected in cells that were irradiated with UVA only. Pretreatment with ferulic acid significantly increased the proliferation and cell cycle progression in HDFs. Moreover, ferulic acid pretreatment produced antioxidant effects such as reduced DCF intensity, and affected SOD1 and CAT mRNA expression. These effects were also demonstrated in the analysis of cell senescence, promoter activity, expression of senescent markers, and DNA repair.
CONCLUSION
These results demonstrate that ferulic acid exerts protective effects on UVA-induced cell damages via anti-oxidant and stress-inducible cellular mechanisms in HDFs.
MeSH Terms
-
Aging
Animals
Antioxidants
Catalase
Cats
Cell Aging
Cell Cycle
Cell Cycle Checkpoints
Cell Death
Complement System Proteins
DNA Repair
Fibroblasts*
Humans*
Luciferases
Matrix Metalloproteinase 1
Oxidative Stress
Reactive Oxygen Species
Real-Time Polymerase Chain Reaction
RNA, Messenger
Skin Aging
Superoxide Dismutase
Ultraviolet Rays
Xeroderma Pigmentosum
Antioxidants
Catalase
Complement System Proteins
Luciferases
Matrix Metalloproteinase 1
RNA, Messenger
Reactive Oxygen Species
Superoxide Dismutase
Figure
Cited by 1 articles
-
Combination of ferulic acid and exercise alleviates menopause symptoms and skin remodeling in ovariectomized rats
Wonyoung Lee, Jinkyung Cho, Seung-Yeon Yoo, Eunmi Park
Nutr Res Pract. 2025;19(1):30-40. doi: 10.4162/nrp.2025.19.1.30.
Reference
-
1. Lee JJ, An S, Kim KB, Heo J, Cho DH, Oh HM, et al. Extract of ettlia sp. YC001 exerts photoprotective effects against UVB irradiation in normal human dermal fibroblasts. J Microbiol Biotechnol. 2016; 26:775–783.
Article2. Faurschou A. Role of tumor necrosis factor-α in the regulation of keratinocyte cell cycle and DNA repair after ultraviolet-B radiation. Dan Med Bull. 2010; 57:B4179.3. Bae S, Kim K, Cha HJ, Choi Y, Shin SH, An IS, et al. Low-dose γ-irradiation induces dual radio-adaptive responses depending on the post-irradiation time by altering microRNA expression profiles in normal human dermal fibroblasts. Int J Mol Med. 2015; 35:227–237.
Article4. Bae S, An IS, An S. Development of a high-throughput screening system for identification of novel reagents regulating DNA damage in human dermal fibroblasts. Acta Pharm. 2015; 65:331–341.
Article5. Mac-Mary S, Sainthillier JM, Jeudy A, Sladen C, Williams C, Bell M, et al. Assessment of cumulative exposure to UVA through the study of asymmetrical facial skin aging. Clin Interv Aging. 2010; 5:277–284.6. Saito Y, Tsuruma K, Ichihara K, Shimazawa M, Hara H. Brazilian green propolis water extract up-regulates the early expression level of HO-1 and accelerates Nrf2 after UVA irradiation. BMC Complement Altern Med. 2015; 15:421.
Article7. Bossi O, Gartsbein M, Leitges M, Kuroki T, Grossman S, Tennenbaum T. UV irradiation increases ROS production via PKCdelta signaling in primary murine fibroblasts. J Cell Biochem. 2008; 105:194–207.
Article8. Sjerobabski-Masnec I, Situm M. Skin aging. Acta Clin Croat. 2010; 49:515–518.9. Zhou BR, Yin HB, Xu Y, Wu D, Zhang ZH, Yin ZQ, et al. Baicalin protects human skin fibroblasts from ultraviolet A radiation-induced oxidative damage and apoptosis. Free Radic Res. 2012; 46:1458–1471.
Article10. Lan CC, Ho PY, Wu CS, Yang RC, Yu HS. LED 590 nm photomodulation reduces UVA-induced metalloproteinase-1 expression via upregulation of antioxidant enzyme catalase. J Dermatol Sci. 2015; 78:125–132.
Article11. Vile GF, Tyrrell RM. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993; 268:14678–14681.
Article12. Varani J, Spearman D, Perone P, Fligiel SE, Datta SC, Wang ZQ, et al. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001; 158:931–942.
Article13. Janicke B, Hegardt C, Krogh M, Onning G, Akesson B, Cirenajwis HM, et al. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr Cancer. 2011; 63:611–622.
Article14. Xu X, Xiao H, Zhao J, Zhao T. Cardioprotective effect of sodium ferulate in diabetic rats. Int J Med Sci. 2012; 9:291–300.
Article15. Roy S, Metya SK, Sannigrahi S, Rahaman N, Ahmed F. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine. 2013; 44:369–379.
Article16. Sgarbossa A, Giacomazza D, di Carlo M. Ferulic acid: a hope for Alzheimer's disease therapy from plants. Nutrients. 2015; 7:5764–5782.
Article17. Ardiansyah , Ohsaki Y, Shirakawa H, Koseki T, Komai M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J Agric Food Chem. 2008; 56:2825–2830.
Article18. Jung EH, Kim SR, Hwang IK, Ha TY. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J Agric Food Chem. 2007; 55:9800–9804.
Article19. Saija A, Tomaino A, Trombetta D, De Pasquale A, Uccella N, Barbuzzi T, et al. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int J Pharm. 2000; 199:39–47.
Article20. Lee MJ, Cha HJ, Lim KM, Lee OK, Bae S, Kim CH, et al. Analysis of the microRNA expression profile of normal human dermal papilla cells treated with 5α-dihydrotestosterone. Mol Med Rep. 2015; 12:1205–1212.
Article21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article22. Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L, et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002; 62:7305–7315.23. Fornace AJ Jr, Nebert DW, Hollander MC, Luethy JD, Papathanasiou M, Fargnoli J, et al. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989; 9:4196–4203.
Article24. Zhou BR, Xu Y, Wu D, Permatasari F, Gao YY, Luo D. Ginsenoside Rg1 protects human fibroblasts against psoralen- and UVA-induced premature senescence through a telomeric mechanism. Arch Dermatol Res. 2012; 304:223–228.
Article25. Chen A, Huang X, Xue Z, Cao D, Huang K, Chen J, et al. The role of p21 in apoptosis, proliferation, cell cycle arrest, and antioxidant activity in UVB-irradiated human HaCaT keratinocytes. Med Sci Monit Basic Res. 2015; 21:86–95.
Article26. Kearsey JM, Coates PJ, Prescott AR, Warbrick E, Hall PA. Gadd45 is a nuclear cell cycle regulated protein which interacts with p21Cip1. Oncogene. 1995; 11:1675–1683.27. Park JM, Choi JY, Yi JM, Chung JW, Leem SH, Koh SS, et al. NDR1 modulates the UV-induced DNA-damage checkpoint and nucleotide excision repair. Biochem Biophys Res Commun. 2015; 461:543–548.
Article28. Lo HL, Nakajima S, Ma L, Walter B, Yasui A, Ethell DW, et al. Differential biologic effects of CPD and 6-4PP UV-induced DNA damage on the induction of apoptosis and cell-cycle arrest. BMC Cancer. 2005; 5:135.
Article29. Chiganças V, Batista LF, Brumatti G, Amarante-Mendes GP, Yasui A, Menck CF. Photorepair of RNA polymerase arrest and apoptosis after ultraviolet irradiation in normal and XPB deficient rodent cells. Cell Death Differ. 2002; 9:1099–1107.
Article30. Chiganças V, Miyaji EN, Muotri AR, de Fátima, Amarante-Mendes GP, Yasui A, et al. Photorepair prevents ultraviolet-induced apoptosis in human cells expressing the marsupial photolyase gene. Cancer Res. 2000; 60:2458–2463.31. You YH, Lee DH, Yoon JH, Nakajima S, Yasui A, Pfeifer GP. Cyclobutane pyrimidine dimers are responsible for the vast majority of mutations induced by UVB irradiation in mammalian cells. J Biol Chem. 2001; 276:44688–44694.
Article32. Jaszewska E, Soin M, Filipek A, Naruszewicz M. UVA-induced ROS generation inhibition by Oenothera paradoxa defatted seeds extract and subsequent cell death in human dermal fibroblasts. J Photochem Photobiol B. 2013; 126:42–46.
Article33. Aroun A, Zhong JL, Tyrrell RM, Pourzand C. Iron, oxidative stress and the example of solar ultraviolet A radiation. Photochem Photobiol Sci. 2012; 11:118–134.
Article34. Maverakis E, Miyamura Y, Bowen MP, Correa G, Ono Y, Goodarzi H. Light, including ultraviolet. J Autoimmun. 2010; 34:J247–J257.
Article35. Saify K, Saadat I, Saadat M. Down-regulation of antioxidant genes in human SH-SY5Y cells after treatment with morphine. Life Sci. 2016; 144:26–29.
Article36. Kohl E, Steinbauer J, Landthaler M, Szeimies RM. Skin ageing. J Eur Acad Dermatol Venereol. 2011; 25:873–884.
Article37. Krutmann J. The role of UVA rays in skin aging. Eur J Dermatol. 2001; 11:170–171.38. Lee KS, Cha HJ, Lee GT, Lee KK, Hong JT, Ahn KJ, et al. Troxerutin induces protective effects against ultraviolet B radiation through the alteration of microRNA expression in human HaCaT keratinocyte cells. Int J Mol Med. 2014; 33:934–942.
Article39. Assefa Z, Van Laethem A, Garmyn M, Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim Biophys Acta. 2005; 1755:90–106.
Article40. Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012; 130:1715–1725.
Article41. Mancuso C, Santangelo R. Ferulic acid: pharmacological and toxicological aspects. Food Chem Toxicol. 2014; 65:185–195.
Article42. Quan T, Qin Z, Xu Y, He T, Kang S, Voorhees JJ, et al. Ultraviolet irradiation induces CYR61/CCN1, a mediator of collagen homeostasis, through activation of transcription factor AP-1 in human skin fibroblasts. J Invest Dermatol. 2010; 130:1697–1706.
Article43. Yang Y, Li S. Dandelion extracts protect human skin fibroblasts from UVB damage and cellular senescence. Oxid Med Cell Longev. 2015; 2015:619560.
Article44. Cho S, Won CH, Lee DH, Lee MJ, Lee S, So SH, et al. Red ginseng root extract mixed with Torilus fructus and Corni fructus improves facial wrinkles and increases type I procollagen synthesis in human skin: a randomized, double-blind, placebo-controlled study. J Med Food. 2009; 12:1252–1259.
Article45. Kim JA, Ahn BN, Kong CS, Kim SK. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br J Dermatol. 2013; 168:968–976.
Article46. Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011; 69:249–256.
Article47. Park SY, Park JY, Kim CH, Kang SU, Kim JH, Bark KM, et al. Effects of xanthium stramarium and psoralea corylifolia extracts combined with UVA1 irradiation on the cell proliferation and TGF-β1 expression of keloid fibroblasts. Ann Dermatol. 2013; 25:304–309.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Behavior of Fibroblasts on a Porous Hyaluronic Acid Incorporated Collagen Matrix
- The Effect of All-Trans-Retinoic Acid and Ursolic Acid on the Ultraviolet A Radiation Induced AP-1 (Fos/Jun) Activity in Cultured Human Dermal Fibroblasts
- Transcriptional Regulation of Proteoglycans and Glycosaminoglycan Chain-synthesizing Glycosyltransferases by UV Irradiation in Cultured Human Dermal Fibroblasts
- Protective actions of Rubus coreanus ethanol extract on collagenous extracellular matrix in ultraviolet-B irradiation-induced human dermal fibroblasts
- Ultraviolet B Downregulated Aquaporin 1 Expression via the MEK/ERK pathway in the Dermal Fibroblasts