J Korean Med Sci.
2011 Mar;26(3):417-424. 10.3346/jkms.2011.26.3.417.
Transcriptional Regulation of Proteoglycans and Glycosaminoglycan Chain-synthesizing Glycosyltransferases by UV Irradiation in Cultured Human Dermal Fibroblasts
- Affiliations
-
- 1Department of Dermatology, Seoul National University College of Medicine, Institute of Dermatological Science, Medical Research Center, Seoul National University, Laboratory of Cutaneous Aging Research, Clinical Research Institute, Seoul National Universi
- KMID: 2157881
- DOI: http://doi.org/10.3346/jkms.2011.26.3.417
Abstract
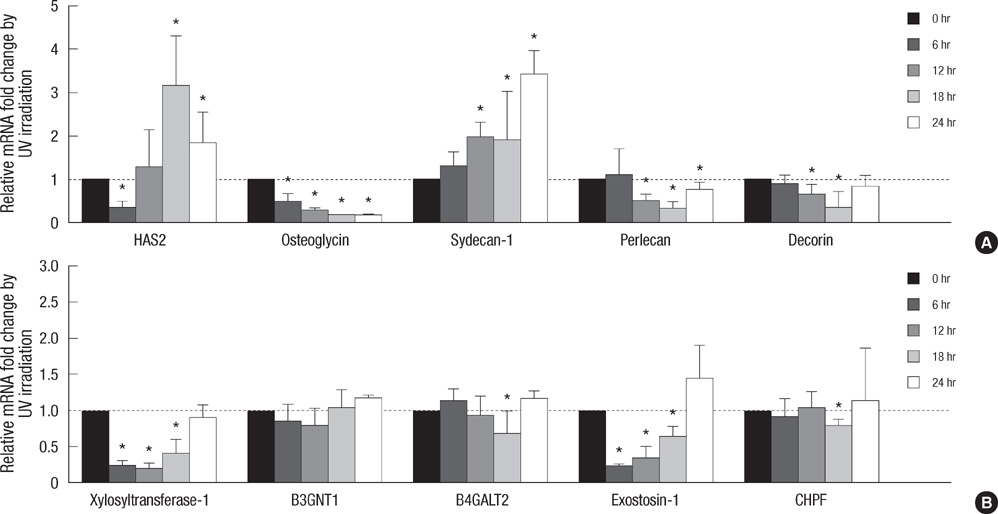
- Various kinds of glycosaminoglycans (GAGs) and proteoglycans (PGs) have been known to be involved in structural and space-filling functions, as well as many physiological regulations in skin. To investigate ultraviolet (UV) radiation-mediated regulation of GAGs and PGs in cultured human dermal fibroblasts, transcriptional changes of many types of PGs and GAG chain-synthesizing enzymes at 18 hr after 75 mJ/cm2 of UV irradiation were examined using quantitative real-time polymerase chain reaction methods. Hyaluronic acid synthase (HAS)-1, -2, and -3 and hyaluronidase-2 mRNA expressions were significantly increased by UV irradiation. Expressions of lumican, fibromodulin, osteoglycin, syndecan-2, perlecan, agrin, versican, decorin, and biglycan were significantly decreased by UV irradiation, while syndecan-1 was increased. Expressions of GAG chain-synthesizing glycosyltransferases, xylosyltransferase-1, beta1,3-glucuronyltransferase-1, beta1,4-galactosyltransferase-2, -4, exostosin-1, chondroitin polymerizing factor, and chondroitin sulfate synthase-3 were significantly reduced, whereas those of beta1,3-galactosyltransferase-6, beta1,4-galactosyltransferase-3, -7, beta-1,3-N-acetylglucosaminyltran sferase-2, and -7 were increased by UV irradiation. Heparanase-1 mRNA expression was increased, but that of heparanase-2 was reduced by UV irradiation. Time-course investigation of representative genes showed consistent results. In conclusion, UV irradiation may increase hyaluronic acid production through HAS induction, and decrease other GAG productions through downregulation of PG core proteins and GAG chain-synthesizing glycosyltransferases in cultured human dermal fibroblasts.
Keyword
MeSH Terms
-
Cell Line
Fibroblasts/metabolism/radiation effects
Gene Expression Regulation/radiation effects
Glucuronosyltransferase/genetics/radiation effects
Glycosaminoglycans/*biosynthesis/chemistry
Glycosyltransferases/genetics/*metabolism
Humans
Hyaluronic Acid/biosynthesis
Hyaluronoglucosaminidase/genetics/radiation effects
Polymerase Chain Reaction
Proteoglycans/*biosynthesis/genetics/radiation effects
RNA, Messenger/analysis/genetics
Skin/*metabolism/radiation effects
Transcription, Genetic/radiation effects
*Ultraviolet Rays
Figure
Reference
-
1. Chung JH. Photoaging in Asians. Photodermatol Photoimmunol Photomed. 2003. 19:109–121.2. Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, Cho KH, Kim KH, Park KC, Eun HC. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001. 117:1218–1224.3. Kim EJ, Kim MK, Jin XJ, Oh JH, Kim JE, Chung JH. Skin aging and photoaging alter fatty acids composition, including 11,14,17-eicosatrienoic acid, in the epidermis of human skin. J Korean Med Sci. 2010. 25:980–983.4. Jin XJ, Kim EJ, Oh IK, Kim YK, Park CH, Chung JH. Prevention of UV-induced skin damages by 11,14,17-eicosatrienoic acid in hairless mice in vivo. J Korean Med Sci. 2010. 25:930–937.5. Seo JY, Lee SH, Youn CS, Choi HR, Rhie GE, Cho KH, Kim KH, Park KC, Eun HC, Chung JH. Ultraviolet radiation increases tropoelastin mRNA expression in the epidermis of human skin in vivo. J Invest Dermatol. 2001. 116:915–919.6. Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: hostassociated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006. 20:9–22.7. Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 2007. 127:687–697.8. Carrino DA, Onnerfjord P, Sandy JD, Cs-Szabo G, Scott PG, Sorrell JM, Heinegård D, Caplan AI. Age-related changes in the proteoglycans of human skin. Specific cleavage of decorin to yield a major catabolic fragment in adult skin. J Biol Chem. 2003. 278:17566–17572.9. Trowbridge JM, Gallo RL. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002. 12:117R–125R.10. Pavão MS, Vilela-Silva AC, Mourão PA. Biosynthesis of chondroitin sulfate: from the early, precursor discoveries to nowadays, genetics approaches. Adv Pharmacol. 2006. 53:117–140.11. Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006. 16:40–51.12. Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998. 141:1277–1286.13. Svensson L, Aszódi A, Reinholt FP, Fässler R, Heinegård D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999. 274:9636–9647.14. Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003. 278:21672–21677.15. Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, Conrad GW. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 2002. 8:407–415.16. Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002. 19:249–255.17. Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002. 17:1180–1189.18. Reinboth B, Hanssen E, Cleary EG, Gibson MA. Molecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J Biol Chem. 2002. 277:3950–3957.19. Nuka S, Zhou W, Henry SP, Gendron CM, Schultz JB, Shinomura T, Johnson J, Wang Y, Keene DR, Ramirez-Solis R, Behringer RR, Young MF, Hook M. Phenotypic characterization of epiphycan-deficient and epiphycan/biglycan double-deficient mice. Osteoarthritis Cartilage. 2010. 18:88–96.20. Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006. 6:633–643.21. Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. The cleavage of biglycan by aggrecanases. Osteoarthritis Cartilage. 2006. 14:1147–1154.22. Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005. 96:488–500.23. Funderburgh JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000. 10:951–958.24. Pang J, Zhang S, Yang P, Hawkins-Lee B, Zhong J, Zhang Y, Ochoa B, Agundez JA, Voelckel MA, Fisher RB, Gu W, Xiong WC, Mei L, She JX, Wang CY. Loss-of-function mutations in HPSE2 cause the autosomal recessive urofacial syndrome. Am J Hum Genet. 2010. 86:957–962.25. Kim MK, Shin JM, Eun HC, Chung JH. The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PLoS One. 2009. 4:e4864.26. Cho S, Kim HH, Lee MJ, Lee S, Park CS, Nam SJ, Han JJ, Kim JW, Chung JH. Phosphatidylserine prevents UV-induced decrease of type I procollagen and increase of MMP-1 in dermal fibroblasts and human skin in vivo. J Lipid Res. 2008. 49:1235–1245.27. Kakizaki I, Itano N, Kimata K, Hanada K, Kon A, Yamaguchi M, Takahashi T, Takagaki K. Up-regulation of hyaluronan synthase genes in cultured human epidermal keratinocytes by UVB irradiation. Arch Biochem Biophys. 2008. 471:85–93.28. Gambichler T, Tomi NS, Skrygan M, Altmeyer P, Kreuter A. Significant decrease of decorin expression in human skin following short-term ultraviolet exposures. J Dermatol Sci. 2007. 45:203–205.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute UV Irradiation Increases Heparan Sulfate Proteoglycan Levels in Human Skin
- Protective actions of Rubus coreanus ethanol extract on collagenous extracellular matrix in ultraviolet-B irradiation-induced human dermal fibroblasts
- Ellagic acid plays a protective role against UV-B-induced oxidative stress by up-regulating antioxidant components in human dermal fibroblasts
- Effects of Helium - Neon Laser Irradiation on Proliferation and collagen Synthesis by Human Dermal Fibroblasts Cultured in a Monolayer and Collagen Lattice
- Effects of Helium - Neon Laser Irradiation on Proliferation and collagen Synthesis by Human Dermal Fibroblasts Cultured in a Monolayer and Collagen Lattice