Allergy Asthma Immunol Res.
2013 Jul;5(4):197-206. 10.4168/aair.2013.5.4.197.
Effects of Interleukin-9 Blockade on Chronic Airway Inflammation in Murine Asthma Models
- Affiliations
-
- 1Department of Internal Medicine, Soonchunhyang University Gumi Hospital, Gumi, Korea.
- 2Department of Microbiology, Ewha Womans University School of Medicine, Seoul, Korea. soyounwoo@ewha.ac.kr
- 3Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2260312
- DOI: http://doi.org/10.4168/aair.2013.5.4.197
Abstract
- PURPOSE
Asthma is a chronic inflammatory disease of the airways associated with structural changes and airway remodeling. Interleukin (IL)-9 has pleiotropic effects on both inflammatory cells and airway structural cells, which are involved in asthma pathogenesis. We evaluated the effects of IL-9 blockade on chronic airway inflammation.
METHODS
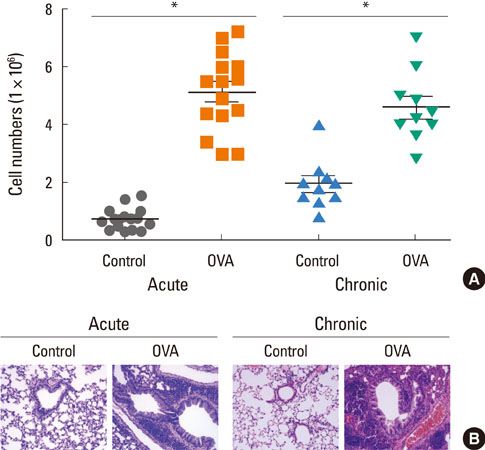
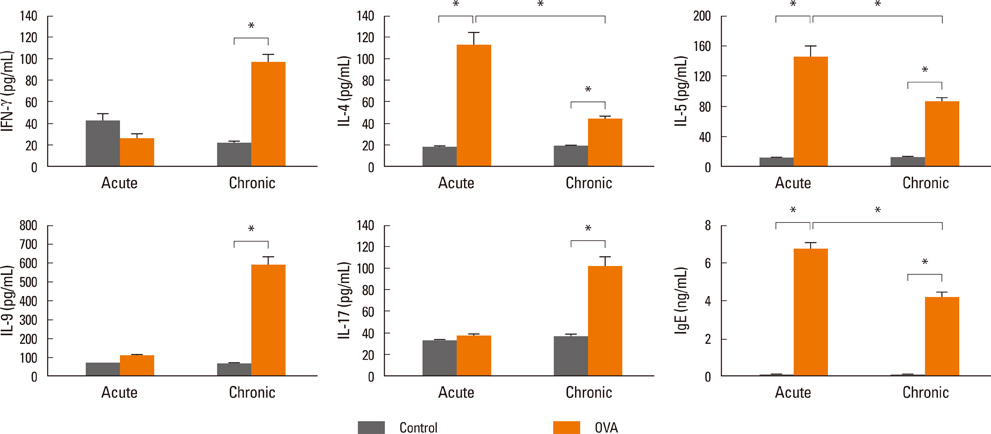
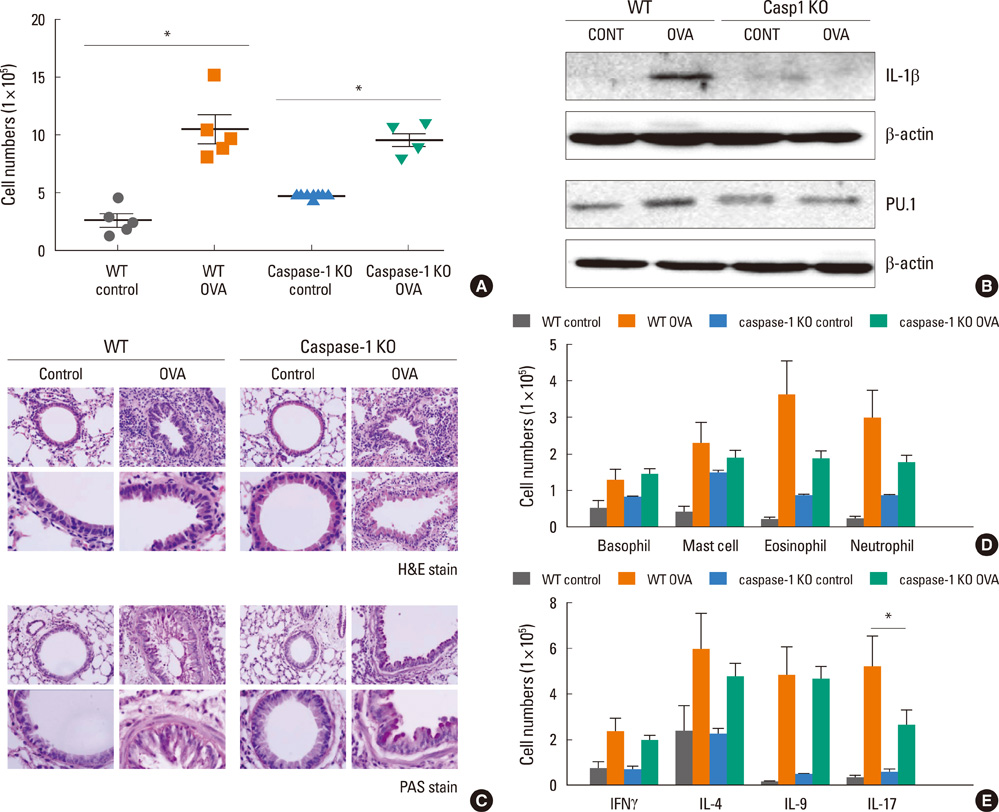
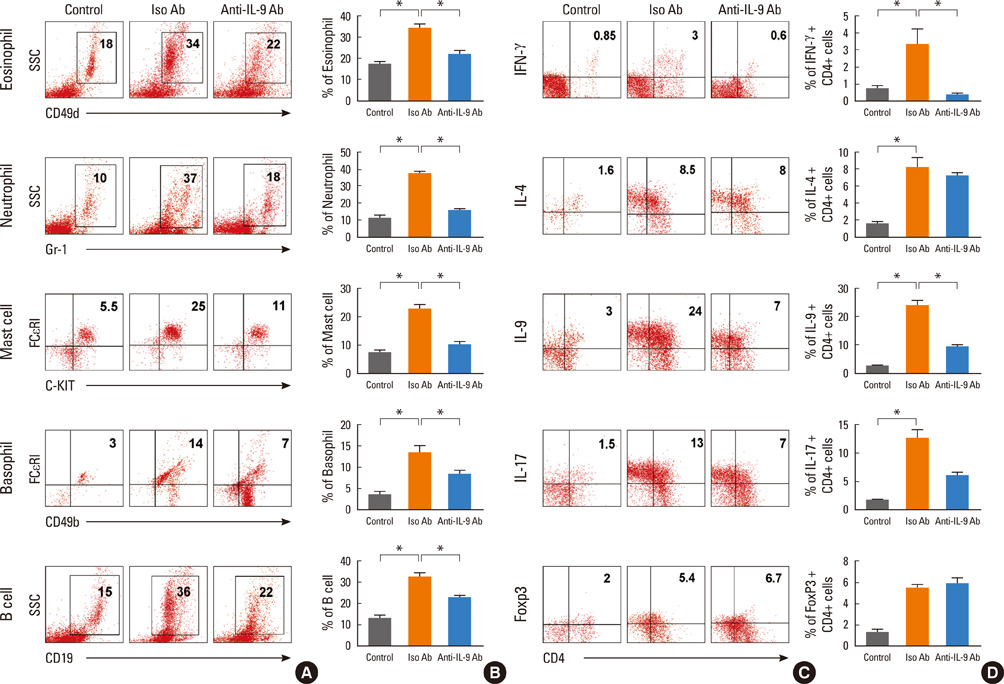
Acute airway inflammation was induced in Balb/c mice using aerosolized ovalbumin (OVA), whereas chronic asthma was induced by OVA exposure for 5 weeks with anti-IL-9 or isotype-matched antibody (Ab) treatment during the OVA challenge. Inflammatory cells in bronchoalveolar lavage fluid (BALF) were counted and lung tissues were stained to detect cellular infiltration, mucus deposition, and collagen accumulation. The levels of interferon (IFN)-gamma, IL-4, IL-5, IL-9, IL-17, and immunoglobulin E (IgE) in BALF were measured using enzyme linked immunosorbent assays, and profiles of inflammatory cells and subsets of T helper (Th) cells were analyzed using flow cytometry.
RESULTS
IL-9, IL-17, and IFN-gamma levels were significantly increased in the chronic group compared to the acute asthma group. However, the number of IL-9-positive cells was not affected, with a decrease in Th17 cells in OVA-challenged caspase-1 knockout mice. Numbers of eosinophils, neutrophils, B cells, mast cells, and Th17 cells decreased after administration of anti-IL-9 Ab. Total IgE, IL-5, IL-9, and IL-17 levels were also lower in the anti-IL-9 group.
CONCLUSIONS
Our results suggest that anti-IL-9 Ab treatment inhibits pulmonary infiltration of inflammatory cells and cytokine production, especially IL-17. These results provide a basis for the use of an anti-IL-9 Ab to combat IL-17-mediated airway inflammation.
MeSH Terms
-
Airway Remodeling
Animals
Asthma
B-Lymphocytes
Bronchoalveolar Lavage Fluid
Collagen
Eosinophils
Immunoglobulin E
Immunoglobulins
Inflammation
Interferons
Interleukin-17
Interleukin-4
Interleukin-5
Interleukin-9
Interleukins
Lung
Mast Cells
Mice
Mice, Knockout
Mucus
Neutrophils
Ovalbumin
Ovum
Th17 Cells
Collagen
Immunoglobulin E
Immunoglobulins
Interferons
Interleukin-17
Interleukin-4
Interleukin-5
Interleukin-9
Interleukins
Ovalbumin
Figure
Cited by 2 articles
-
Magnolol exerts anti-asthmatic effects by regulating Janus kinase-signal transduction and activation of transcription and Notch signaling pathways and modulating Th1/Th2/Th17 cytokines in ovalbumin-sensitized asthmatic mice
Qi Huang, Lele Han, Rong Lv, Ling Ling
Korean J Physiol Pharmacol. 2019;23(4):251-261. doi: 10.4196/kjpp.2019.23.4.251.Anti-Interleukin-9 Antibody Increases the Effect of Allergen-Specific Immunotherapy in Murine Allergic Rhinitis
Ji-Hyeon Shin, Do Hyun Kim, Boo-Young Kim, Sung Won Kim, Se Hwan Hwang, Joohyung Lee, Soo Whan Kim
Allergy Asthma Immunol Res. 2017;9(3):237-246. doi: 10.4168/aair.2017.9.3.237.
Reference
-
1. Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention [Internet]. Vancouver (WA): GINA;2011. Available from: http://www.ginasthma.org/.2. Yamauchi K, Inoue H. Airway remodeling in asthma and irreversible airflow limitation-ECM deposition in airway and possible therapy for remodeling. Allergol Int. 2007; 56:321–329.3. Yamauchi K. Airway remodeling in asthma and its influence on clinical pathophysiology. Tohoku J Exp Med. 2006; 209:75–87.4. Durrani SR, Viswanathan RK, Busse WW. What effect does asthma treatment have on airway remodeling? Current perspectives. J Allergy Clin Immunol. 2011; 128:439–448.5. Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, Jeffery PK. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003; 167:78–82.6. Cho KA, Suh JW, Sohn JH, Park JW, Lee H, Kang JL, Woo SY, Cho YJ. IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L429–L440.7. Buc M, Dzurilla M, Vrlik M, Bucova M. Immunopathogenesis of bronchial asthma. Arch Immunol Ther Exp (Warsz). 2009; 57:331–344.8. Kaiko GE, Foster PS. New insights into the generation of Th2 immunity and potential therapeutic targets for the treatment of asthma. Curr Opin Allergy Clin Immunol. 2011; 11:39–45.9. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009; 360:985–993.10. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009; 360:973–984.11. Corren J. Cytokine inhibition in severe asthma: current knowledge and future directions. Curr Opin Pulm Med. 2011; 17:29–33.12. Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006; 354:697–708.13. Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011; 186:3283–3288.14. Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010; 11:527–534.15. Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4 memory T cells can become CD4+IL-9+ T cells. PLoS One. 2010; 5:e8706.16. Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, Dehzad N, Becker M, Stassen M, Steinborn A, Lohoff M, Schild H, Schmitt E, Bopp T. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010; 33:192–202.17. McLane MP, Haczku A, van de Rijn M, Weiss C, Ferrante V, MacDonald D, Renauld JC, Nicolaides NC, Holroyd KJ, Levitt RC. Interleukin-9 promotes allergen-induced eosinophilic inflammation and airway hyperresponsiveness in transgenic mice. Am J Respir Cell Mol Biol. 1998; 19:713–720.18. Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, Meyers DA, Levitt RC. Genetic susceptibility to asthma--bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995; 333:894–900.19. Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, Zhou T, Kleeberger SR, Buetow KH, Levitt RC. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci U S A. 1997; 94:13175–13180.20. Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, Kim YE, Shin JA, Park CS, Park JW, Park TK, Lee JH, Seo BF, Kim KD, Kim ES, Lee DH, Lee SK, Lee SK. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006; 12:574–579.21. Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006; 203:1685–1691.22. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135.23. Erpenbeck VJ, Hohlfeld JM, Discher M, Krentel H, Hagenberg A, Braun A, Krug N. Increased expression of interleukin-9 messenger RNA after segmental allergen challenge in allergic asthmatics. Chest. 2003; 123:370S.24. Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, Nicolaides NC, Holroyd KJ, Tsicopoulos A, Lafitte JJ, Wallaert B, Hamid QA. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol. 2000; 105:108–115.25. Bhathena PR, Comhair SA, Holroyd KJ, Erzurum SC. Interleukin-9 receptor expression in asthmatic airways In vivo. Lung. 2000; 178:149–160.26. Abdelilah S, Latifa K, Esra N, Cameron L, Bouchaib L, Nicolaides N, Levitt R, Hamid Q. Functional expression of IL-9 receptor by human neutrophils from asthmatic donors: role in IL-8 release. J Immunol. 2001; 166:2768–2774.27. Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J Clin Invest. 2002; 109:29–39.28. Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000; 13:573–583.29. Cheng G, Arima M, Honda K, Hirata H, Eda F, Yoshida N, Fukushima F, Ishii Y, Fukuda T. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am J Respir Crit Care Med. 2002; 166:409–416.30. Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, Kiener PA, Kolbeck R, Lloyd CM, Coyle AJ, Humbles AA. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011; 183:865–875.31. Doherty TA, Soroosh P, Broide DH, Croft M. CD4+ cells are required for chronic eosinophilic lung inflammation but not airway remodeling. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L229–L235.32. Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009; 127:450–458.33. Parker JM, Oh CK, La Force C, Miller SD, Pearlman DS, Le C, Robbie GJ, White WI, White B, Molfino NA. MEDI-528 Clinical Trials Group. Safety profile and clinical activity of multiple subcutaneous doses of MEDI-528, a humanized anti-interleukin-9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulm Med. 2011; 11:14.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model
- Role of IL-23 and Th17 Cells in Airway Inflammation in Asthma
- The Effectiveness of Pimecrolimus in Airway Inflammation and Bronchial Hyperresponsiveness in Murine Asthma Model
- Effect of DHEA on airway hyperresponsiveness and inflammation in murine model of asthma