Allergy Asthma Immunol Res.
2014 Nov;6(6):548-557. 10.4168/aair.2014.6.6.548.
Oral Lovastatin Attenuates Airway Inflammation and Mucus Secretion in Ovalbumin-Induced Murine Model of Asthma
- Affiliations
-
- 1Department of Nursing, Chang Gung University of Science and Technology, Tao-Yuan, Taiwan.
- 2Research Center for Industry of Human Ecology, Chang Gung University of Science and Technology, Kwei-Shan, Tao-Yuan, Taiwan.
- 3Department of Microbiology and Immunology, Graduate Institute of Biomedical Sciences, Chang Gung University, Kwei-Shan, Tao-Yuan, Taiwan.
- 4Department of Nutrition and Health Sciences, Chang Gung University of Science and Technology, Kwei-Shan, Tao-Yuan, Taiwan.
- 5Graduate Institute of Health Industry Technology, Chang Gung University of Science and Technology, Kwei-Shan, Tao-Yuan, Taiwan.
- 6School of Traditional Chinese Medicine, Chang Gung University, Kwei-Shan, Tao-Yuan, Taiwan. shen5421@yahoo.com.tw
- 7Center for Traditional Chinese Medicine, Chang Gung Memorial Hospital at Lin-Kuo, Tao-Yuan, Taiwan.
- KMID: 2260178
- DOI: http://doi.org/10.4168/aair.2014.6.6.548
Abstract
- PURPOSE
Lovastatin is an effective inhibitor of cholesterol synthesis. A previous study demonstrated that lovastatin can also suppress airway hyperresponsiveness (AHR) in murine model of asthma. We aimed to investigate the effect of lovastatin on mucus secretion and inflammation-associated gene expression in the lungs of murine model of asthma.
METHODS
Female BALB/c mice were sensitized and challenged with ovalbumin (OVA) by intraperitoneal injection, and orally administered lovastatin from days 14 to 27 post-injection. Gene expression in lung tissues was analyzed using real-time polymerase chain reaction. AHR and goblet cell hyperplasia were also examined. BEAS-2B human bronchial epithelial cells were used to evaluate the effect of lovastatin on the expression of cell adhesion molecules, chemokines, and proinflammatory cytokines in vitro.
RESULTS
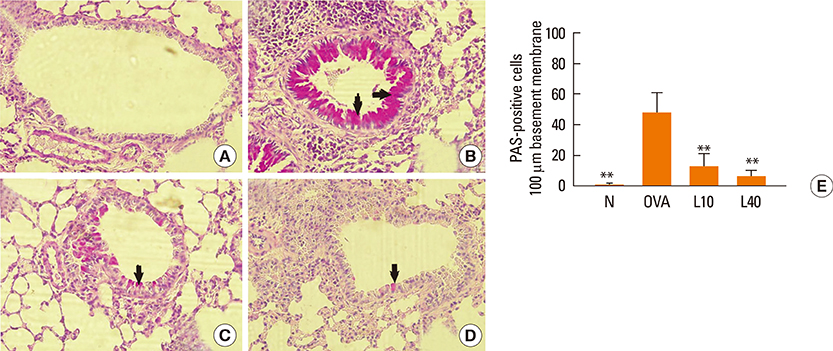
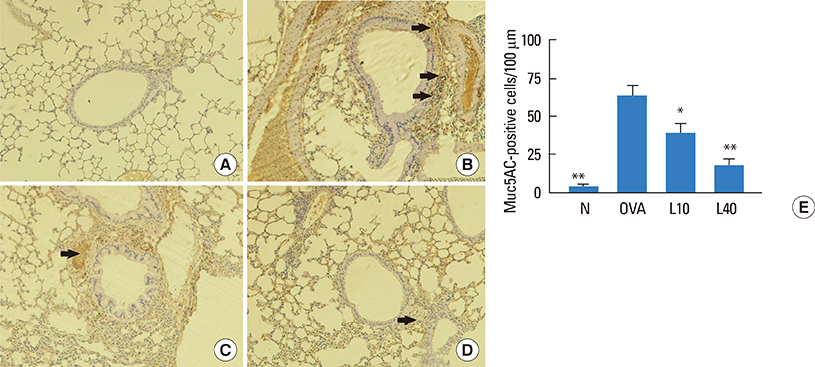
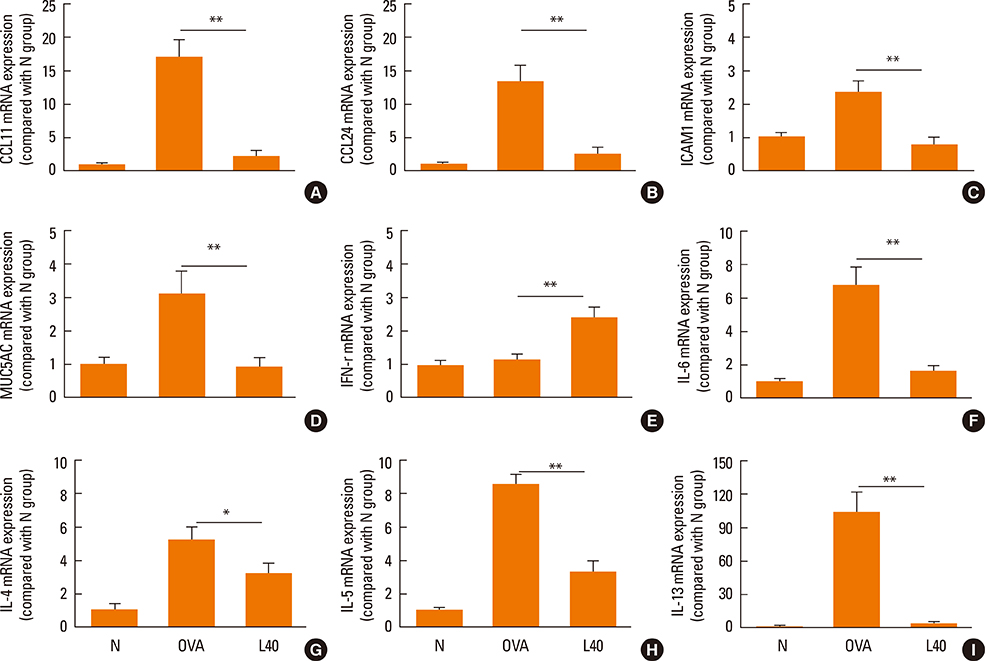
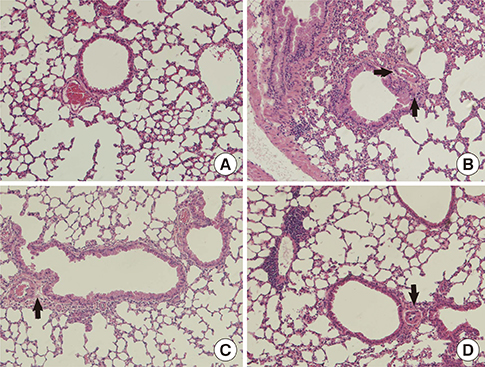
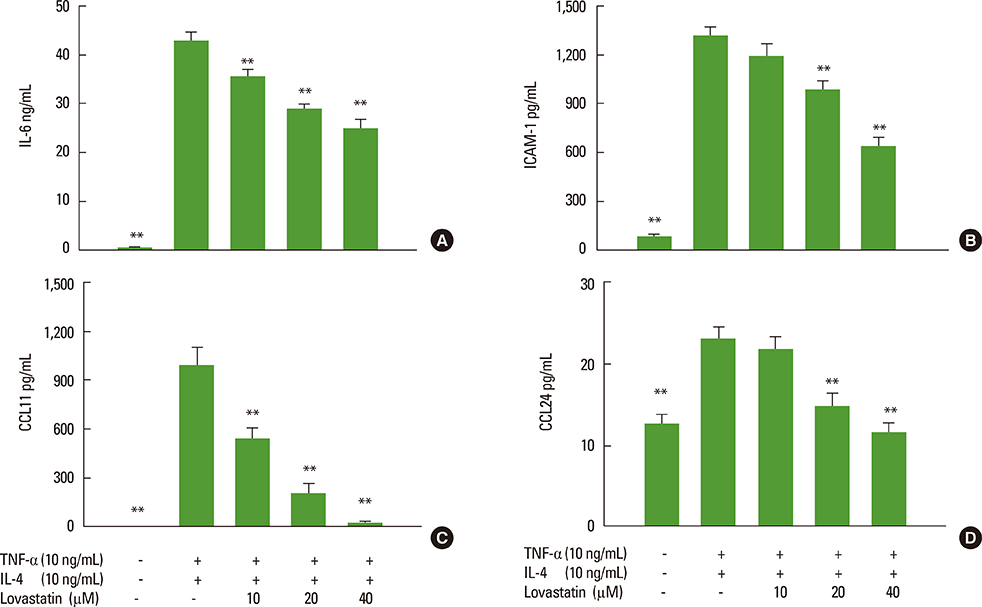
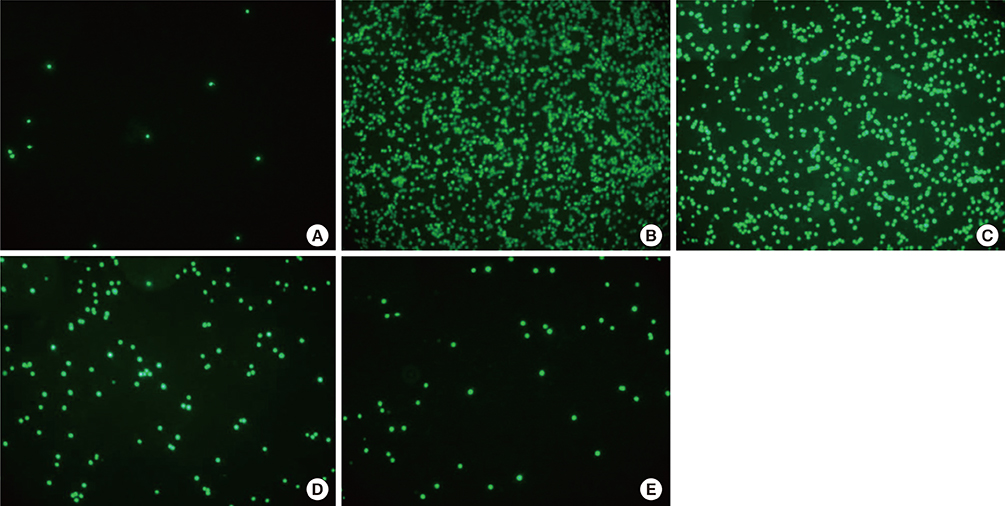
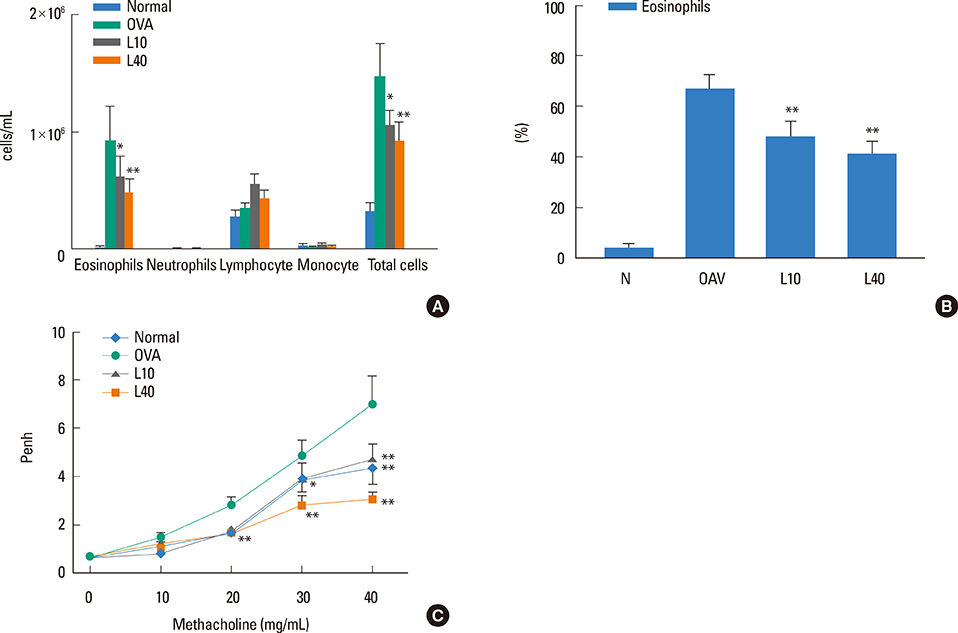
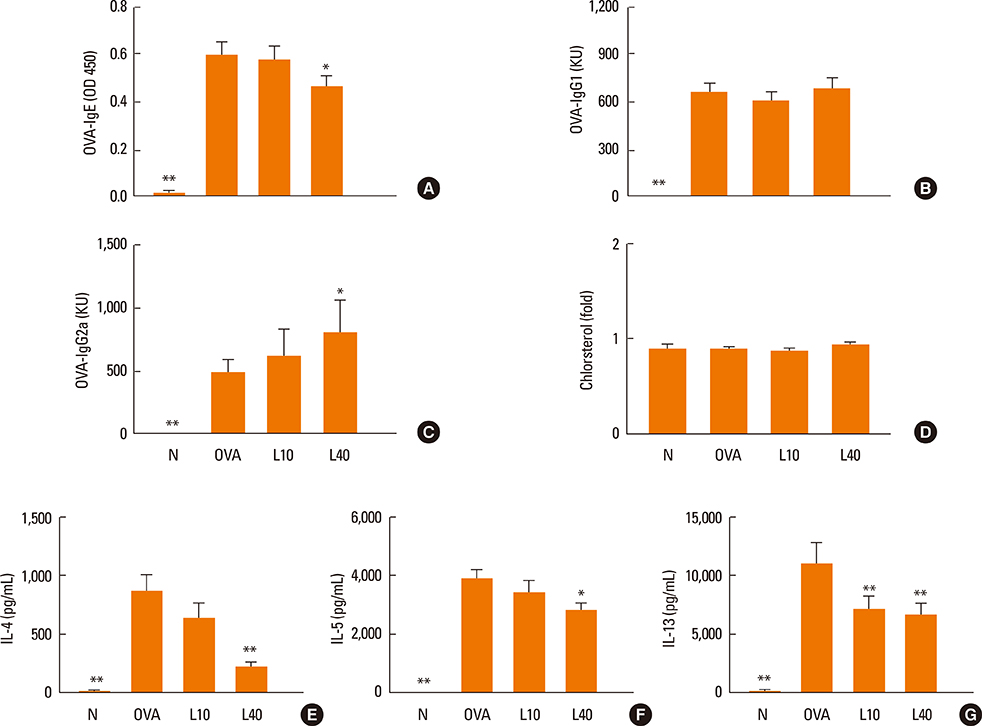
We showed that lovastatin inhibits the expression of Th2-associated genes, including eotaxins and adhesion molecules, in the lungs of murine model of asthma. Mucin 5AC expression, eosinophil infiltration and goblet cell hyperplasia were significantly decreased in the lung tissue of murine model of asthma treated with lovastatin. Furthermore, lovastatin inhibited AHR and expression of Th2-associated cytokines in bronchoalveolar lavage fluid. However, a high dose (40 mg/kg) of lovastatin was required to decrease specific IgE to OVA levels in serum, and suppress the expression of Th2-associated cytokines in splenocytes. Activated BEAS-2B cells treated with lovastatin exhibited reduced IL-6, eotaxins (CCL11 and CCL24), and intercellular adhesion molecule-1 protein expression. Consistent with this, lovastatin also suppressed the ability of HL-60 cells to adhere to inflammatory BEAS-2B cells.
CONCLUSIONS
These data suggest that lovastatin suppresses mucus secretion and airway inflammation by inhibiting the production of eotaxins and Th2 cytokines in murine model of asthma.
Keyword
MeSH Terms
-
Animals
Asthma*
Bronchoalveolar Lavage Fluid
Cell Adhesion Molecules
Chemokines
Cholesterol
Cytokines
Eosinophils
Epithelial Cells
Female
Gene Expression
Goblet Cells
HL-60 Cells
Humans
Hyperplasia
Immunoglobulin E
Inflammation*
Injections, Intraperitoneal
Intercellular Adhesion Molecule-1
Interleukin-6
Lovastatin*
Lung
Mice
Mucin 5AC
Mucus*
Ovalbumin
Ovum
Real-Time Polymerase Chain Reaction
Cell Adhesion Molecules
Chemokines
Cholesterol
Cytokines
Immunoglobulin E
Intercellular Adhesion Molecule-1
Interleukin-6
Lovastatin
Mucin 5AC
Ovalbumin
Figure
Cited by 1 articles
-
The Effects of Statin Medications on Postoperative Outcomes in Patients With Chronic Rhinosinusitis
Hye Kyu Min, Hyun Ji Lee, Jeon Gang Doo, Sung Wan Kim, Jin-Young Min
Clin Exp Otorhinolaryngol. 2023;16(1):95-97. doi: 10.21053/ceo.2022.00780.
Reference
-
1. Poon AH, Eidelman DH, Martin JG, Laprise C, Hamid Q. Pathogenesis of severe asthma. Clin Exp Allergy. 2012; 42:625–637.2. Agrawal DK, Shao Z. Pathogenesis of allergic airway inflammation. Curr Allergy Asthma Rep. 2010; 10:39–48.3. Kim YM, Kim YS, Jeon SG, Kim YK. Immunopathogenesis of allergic asthma: more than the Th2 hypothesis. Allergy Asthma Immunol Res. 2013; 5:189–196.4. Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol. 2008; 70:405–429.5. Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008; 70:487–512.6. Kim KC. Role of epithelial mucins during airway infection. Pulm Pharmacol Ther. 2012; 25:415–419.7. Suh DI, Koh YY. Relationship between atopy and bronchial hyperresponsiveness. Allergy Asthma Immunol Res. 2013; 5:181–188.8. Castro-Rodriguez JA, Rodrigo GJ. A systematic review of long-acting beta2-agonists versus higher doses of inhaled corticosteroids in asthma. Pediatrics. 2012; 130:e650–e657.9. Barnes PJ. Corticosteroids: the drugs to beat. Eur J Pharmacol. 2006; 533:2–14.10. Afshar R, Medoff BD, Luster AD. Allergic asthma: a tale of many T cells. Clin Exp Allergy. 2008; 38:1847–1857.11. Georas SN, Guo J, De Fanis U, Casolaro V. T-helper cell type-2 regulation in allergic disease. Eur Respir J. 2005; 26:1119–1137.12. Koch CG. Statin therapy. Curr Pharm Des. 2012; 18:6284–6290.13. Tiwari R, Pathak K. Statins therapy: a review on conventional and novel formulation approaches. J Pharm Pharmacol. 2011; 63:983–998.14. Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012; 18:1519–1530.15. Silva D, Couto M, Delgado L, Moreira A. A systematic review of statin efficacy in asthma. J Asthma. 2012; 49:885–894.16. Ahmad T, Mabalirajan U, Sharma A, Aich J, Makhija L, Ghosh B, Agrawal A. Simvastatin improves epithelial dysfunction and airway hyperresponsiveness: from asymmetric dimethyl-arginine to asthma. Am J Respir Cell Mol Biol. 2011; 44:531–539.17. Liou CJ, Huang WC. Dehydroepiandrosterone suppresses eosinophil infiltration and airway hyperresponsiveness via modulation of chemokines and Th2 cytokines in ovalbumin-sensitized mice. J Clin Immunol. 2011; 31:656–665.18. Shen JJ, Chiang MS, Kuo ML, Leu YL, Hwang TL, Liou CJ, Huang WC. Partially purified extract and viscolin from Viscum coloratum attenuate airway inflammation and eosinophil infiltration in ovalbumin-sensitized mice. J Ethnopharmacol. 2011; 135:646–653.19. Huang WC, Liou CJ. Dietary acacetin reduces airway hyperresponsiveness and eosinophil infiltration by modulating eotaxin-1 and th2 cytokines in a mouse model of asthma. Evid Based Complement Alternat Med. 2012; 2012:910520.20. Liou CJ, Huang WC, Kuo ML, Yang RC, Shen JJ. Long-term oral administration of Gynostemma pentaphyllum extract attenuates airway inflammation and Th2 cell activities in ovalbumin-sensitized mice. Food Chem Toxicol. 2010; 48:2592–2598.21. Lin CC, Liou CJ, Chiang CY, Huang WY, Huang WC. Danggui Buxue Tang attenuates eosinophil infiltration and airway hyperresponsiveness in asthmatic mice. Ann Allergy Asthma Immunol. 2011; 107:501–509.22. Chuang KP, Tsai WS, Wang YJ, Shieh CC. Superoxide activates very late antigen-4 on an eosinophil cell line and increases cellular binding to vascular cell adhesion molecule-1. Eur J Immunol. 2003; 33:645–655.23. Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008; 38:872–897.24. Proud D, Leigh R. Epithelial cells and airway diseases. Immunol Rev. 2011; 242:186–204.25. Poynter ME. Airway epithelial regulation of allergic sensitization in asthma. Pulm Pharmacol Ther. 2012; 25:438–446.26. Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006; 24:147–174.27. Chiba Y, Arima J, Sakai H, Misawa M. Lovastatin inhibits bronchial hyperresponsiveness by reducing RhoA signaling in rat allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2008; 294:L705–L713.28. Chen L, Grabowski KA, Xin JP, Coleman J, Huang Z, Espiritu B, Alkan S, Xie HB, Zhu Y, White FA, Clancy J Jr, Huang H. IL-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. J Immunol. 2004; 172:2059–2066.29. Kips JC. Cytokines in asthma. Eur Respir J Suppl. 2001; 34:24s–33s.30. Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol. 2004; 31:62–68.31. Leigh R, Ellis R, Wattie J, Donaldson DD, Inman MD. Is interleukin-13 critical in maintaining airway hyperresponsiveness in allergen-challenged mice? Am J Respir Crit Care Med. 2004; 170:851–856.32. Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy. 2011; 41:1747–1756.33. Xu L, Dong XW, Shen LL, Li FF, Jiang JX, Cao R, Yao HY, Shen HJ, Sun Y, Xie QM. Simvastatin delivery via inhalation attenuates airway inflammation in a murine model of asthma. Int Immunopharmacol. 2012; 12:556–564.34. Chiba Y, Sato S, Misawa M. Lovastatin inhibits antigen-induced airway eosinophilia without affecting the production of inflammatory mediators in mice. Inflamm Res. 2009; 58:363–369.35. Capra V, Rovati GE. Rosuvastatin inhibits human airway smooth muscle cells mitogenic response to eicosanoid contractile agents. Pulm Pharmacol Ther. 2014; 27:10–16.36. Imamura M, Okunishi K, Ohtsu H, Nakagome K, Harada H, Tanaka R, Yamamoto K, Dohi M. Pravastatin attenuates allergic airway inflammation by suppressing antigen sensitisation, interleukin 17 production and antigen presentation in the lung. Thorax. 2009; 64:44–49.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dexamethasone on Airway Hyperresponsiveness and Airway Inflammation in Ovalbumin-induced Murine Asthma Model
- Effect of Route of Airway Allergen Challenge on Airway Inflammation and Hyperresponsiveness in Mouse Asthma Model
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- Effects of Korean Red Ginseng Extracts on Airway Hyperresponsiveness and Inflammation in a Murine Asthma Model
- MicroRNA-21 inhibition attenuates airway inflammation and remodelling by modulating the transforming growth factor β–Smad7 pathway