Endocrinol Metab.
2015 Dec;30(4):593-603. 10.3803/EnM.2015.30.4.593.
Selective Mitochondrial Uptake of MKT-077 Can Suppress Medullary Thyroid Carcinoma Cell Survival In Vitro and In Vivo
- Affiliations
-
- 1Department of Biochemistry, Medical College of Wisconsin, Milwaukee, WI, USA. jipark@mcw.edu
- KMID: 2169670
- DOI: http://doi.org/10.3803/EnM.2015.30.4.593
Abstract
- BACKGROUND
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor mainly caused by mutations in the rearranged during transfection (RET) proto-oncogene. Not all patients with progressive MTC respond to current therapy inhibiting RET, demanding additional therapeutic strategies. We recently demonstrated that disrupting mitochondrial metabolism using a mitochondria-targeted agent or by depleting a mitochondrial chaperone effectively suppressed human MTC cells in culture and in mouse xenografts by inducing apoptosis and RET downregulation. These observations led us to hypothesize that mitochondria are potential therapeutic targets for MTC. This study further tests this hypothesis using1-ethyl-2-[[3-ethyl-5-(3-methylbenzothiazolin-2-yliden)]-4-oxothiazolidin-2-ylidenemethyl] pyridinium chloride (MKT-077), a water-soluble rhodocyanine dye analogue, which can selectively accumulate in mitochondria.
METHODS
The effects of MKT-077 on cell proliferation, survival, expression of RET and tumor protein 53 (TP53), and mitochondrial activity were determined in the human MTC lines in culture and in mouse xenografts.
RESULTS
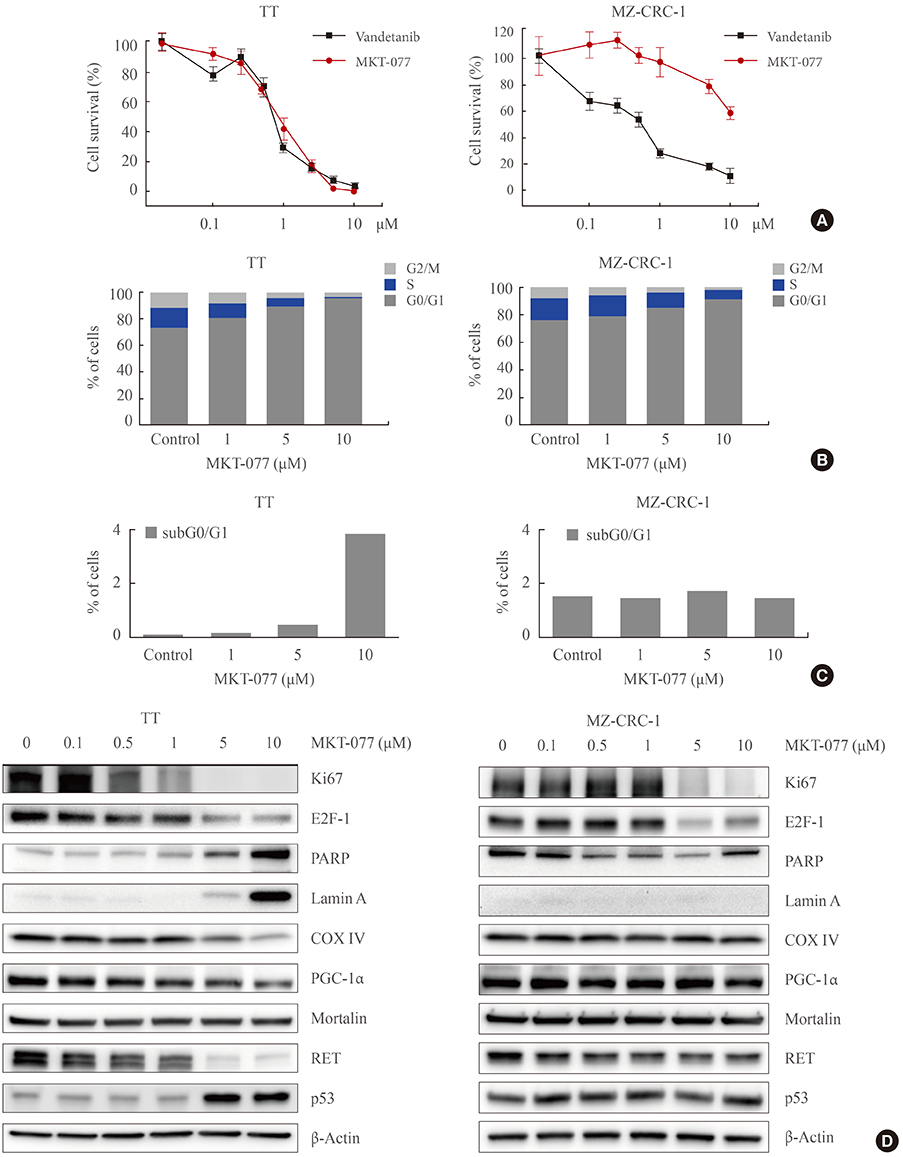
MKT-077 induced cell cycle arrest in TT and MZ-CRC-1. Intriguingly, MKT-077 also induced RET downregulation and strong cell death responses in TT cells, but not in MZ-CRC-1 cells. This discrepancy was mainly due to the difference between the capacities of these cell lines to retain MKT-077 in mitochondria. The cytotoxicity of MKT-077 in TT cells was mainly attributed to oxidative stress while being independent of TP53. MKT-077 also effectively suppressed tumor growth of TT xenografts.
CONCLUSION
MKT-077 can suppress cell survival of certain MTC subtypes by accumulating in mitochondria and interfering with mitochondrial activity although it can also suppress cell proliferation via other mechanisms. These results consistently support the hypothesis that mitochondrial targeting has therapeutic potential for MTC.
Keyword
MeSH Terms
Figure
Reference
-
1. Tuttle RM, Ball DW, Byrd D, Daniels GH, Dilawari RA, Doherty GM, et al. Medullary carcinoma. J Natl Compr Canc Netw. 2010; 8:512–530.2. Agrawal N, Jiao Y, Sausen M, Leary R, Bettegowda C, Roberts NJ, et al. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab. 2013; 98:E364–E369.3. Boichard A, Croux L, Al Ghuzlan A, Broutin S, Dupuy C, Leboulleux S, et al. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab. 2012; 97:E2031–E2035.4. Ciampi R, Mian C, Fugazzola L, Cosci B, Romei C, Barollo S, et al. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid. 2013; 23:50–57.5. Ichihara M, Murakumo Y, Takahashi M. RET and neuroendocrine tumors. Cancer Lett. 2004; 204:197–211.6. Degrauwe N, Sosa JA, Roman S, Deshpande HA. Vandetanib for the treatment of metastatic medullary thyroid cancer. Clin Med Insights Oncol. 2012; 6:243–252.7. Nagilla M, Brown RL, Cohen EE. Cabozantinib for the treatment of advanced medullary thyroid cancer. Adv Ther. 2012; 29:925–934.8. Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012; 30:134–141.9. Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012; 21:297–308.10. Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. 2014; 14:709–721.11. Don AS, Hogg PJ. Mitochondria as cancer drug targets. Trends Mol Med. 2004; 10:372–378.12. Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015; 11:9–15.13. Starenki D, Park JI. Mitochondria-targeted nitroxide, Mito-CP, suppresses medullary thyroid carcinoma cell survival in vitro and in vivo. J Clin Endocrinol Metab. 2013; 98:1529–1540.14. Starenki D, Hong SK, Lloyd RV, Park JI. Mortalin (GRP75/HSPA9) upregulation promotes survival and proliferation of medullary thyroid carcinoma cells. Oncogene. 2015; 34:4624–4634.15. Koya K, Li Y, Wang H, Ukai T, Tatsuta N, Kawakami M, et al. MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res. 1996; 56:538–543.16. Chiba Y, Kubota T, Watanabe M, Matsuzaki SW, Otani Y, Teramoto T, et al. MKT-077, localized lipophilic cation: antitumor activity against human tumor xenografts serially transplanted into nude mice. Anticancer Res. 1998; 18(2A):1047–1052.17. Propper DJ, Braybrooke JP, Taylor DJ, Lodi R, Styles P, Cramer JA, et al. Phase I trial of the selective mitochondrial toxin MKT077 in chemo-resistant solid tumours. Ann Oncol. 1999; 10:923–927.18. Rousaki A, Miyata Y, Jinwal UK, Dickey CA, Gestwicki JE, Zuiderweg ER. Allosteric drugs: the interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J Mol Biol. 2011; 411:614–632.19. Arthan D, Hong SK, Park JI. Leukemia inhibitory factor can mediate Ras/Raf/MEK/ERK-induced growth inhibitory signaling in medullary thyroid cancer cells. Cancer Lett. 2010; 297:31–41.20. Park JI, Strock CJ, Ball DW, Nelkin BD. The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine-paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway. Mol Cell Biol. 2003; 23:543–554.21. Park JI, Strock CJ, Ball DW, Nelkin BD. Interleukin-1beta can mediate growth arrest and differentiation via the leukemia inhibitory factor/JAK/STAT pathway in medullary thyroid carcinoma cells. Cytokine. 2005; 29:125–134.22. Hong SK, Yoon S, Moelling C, Arthan D, Park JI. Noncatalytic function of ERK1/2 can promote Raf/MEK/ERK-mediated growth arrest signaling. J Biol Chem. 2009; 284:33006–33018.23. Wu PK, Hong SK, Veeranki S, Karkhanis M, Starenki D, Plaza JA, et al. A mortalin/HSPA9-mediated switch in tumor-suppressive signaling of Raf/MEK/extracellular signal-regulated kinase. Mol Cell Biol. 2013; 33:4051–4067.24. Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003; 33:401–406.25. de Groot JW, Plaza Menacho I, Schepers H, Drenth-Diephuis LJ, Osinga J, Plukker JT, et al. Cellular effects of imatinib on medullary thyroid cancer cells harboring multiple endocrine neoplasia type 2A and 2B associated RET mutations. Surgery. 2006; 139:806–814.26. Morisi R, Celano M, Tosi E, Schenone S, Navarra M, Ferretti E, et al. Growth inhibition of medullary thyroid carcinoma cells by pyrazolo-pyrimidine derivates. J Endocrinol Invest. 2007; 30:RC31–RC34.27. Plaza-Menacho I, Mologni L, Sala E, Gambacorti-Passerini C, Magee AI, Links TP, et al. Sorafenib functions to potently suppress RET tyrosine kinase activity by direct enzymatic inhibition and promoting RET lysosomal degradation independent of proteasomal targeting. J Biol Chem. 2007; 282:29230–29240.28. Rosen A, Casciola-Rosen L. Macromolecular substrates for the ICE-like proteases during apoptosis. J Cell Biochem. 1997; 64:50–54.29. Modica-Napolitano JS, Aprille JR. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev. 2001; 49:63–70.30. Chunta JL, Vistisen KS, Yazdi Z, Braun RD. Uptake rate of cationic mitochondrial inhibitor MKT-077 determines cellular oxygen consumption change in carcinoma cells. PLoS One. 2012; 7:e37471.31. Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011; 50:98–115.32. Wadhwa R, Sugihara T, Yoshida A, Nomura H, Reddel RR, Simpson R, et al. Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res. 2000; 60:6818–6821.33. Lu WJ, Lee NP, Kaul SC, Lan F, Poon RT, Wadhwa R, et al. Mortalin-p53 interaction in cancer cells is stress dependent and constitutes a selective target for cancer therapy. Cell Death Differ. 2011; 18:1046–1056.34. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998; 281:1309–1312.35. Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007; 401:1–11.36. Kalyanaraman B. Oxidative chemistry of fluorescent dyes: implications in the detection of reactive oxygen and nitrogen species. Biochem Soc Trans. 2011; 39:1221–1225.37. Klein P, Muller-Rischart AK, Motori E, Schonbauer C, Schnorrer F, Winklhofer KF, et al. Ret rescues mitochondrial morphology and muscle degeneration of Drosophila Pink1 mutants. EMBO J. 2014; 33:341–355.38. Kaul SC, Reddel RR, Mitsui Y, Wadhwa R. An N-terminal region of mot-2 binds to p53 in vitro. Neoplasia. 2001; 3:110–114.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent Medullay and Papillary Carcinoma of the Thyroid
- Medullary Thyroid Carcinoma with Normal Calcitonin Level
- Medullary Carcinoma of Thyroid Gland with Co-existing Papillary Carcinoma
- Concurrent Papillary and Medullary Carcinoma of the Thyroid Gland
- Concurrent Medullary Thyroid Carcinoma and Primary Thyroid Lymphoma (Diffuse Large B Cell Lymphoma): the First Case Report