Allergy Asthma Immunol Res.
2015 Jul;7(4):393-403. 10.4168/aair.2015.7.4.393.
Der p2 Internalization by Epithelium Synergistically Augments Toll-like Receptor-Mediated Proinflammatory Signaling
- Affiliations
-
- 1Center for Translational Medicine, Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan.
- 2Department of Bioindustry Technology, Da-Yeh University, Changhua, Taiwan.
- 3Department of Medical Technology, Jen-Teh Junior College of Medicine, Nursing and Management, Miaoli, Taiwan.
- 4Instrumentation Center, Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan.
- 5Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan. jawji@vghtc.gov.tw
- 6Institute of Biomedical Sciences, National Chung-Hsing University, Taichung, Taiwan.
- 7Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan.
- KMID: 2166687
- DOI: http://doi.org/10.4168/aair.2015.7.4.393
Abstract
- PURPOSE
House-dust-mite (HDM) major allergen Der p2 shares homology and function with Toll-like receptor (TLR) signaling protein myeloid differentiation-2 (MD2) and may lead to airway inflammation. Should Der p2 be internalized by human airway epithelium, it has the theoretical propensity to potentiate epithelium activation. This study aimed to demonstrate the internalization of Der p2 by airway epithelium and to investigate the effects of Der p2 on MD2 expression and epithelium activation.
METHODS
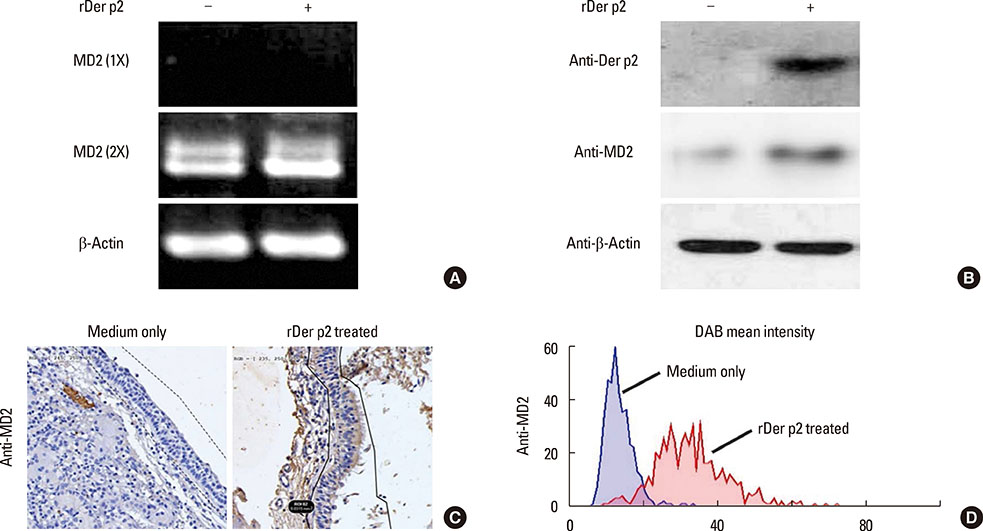
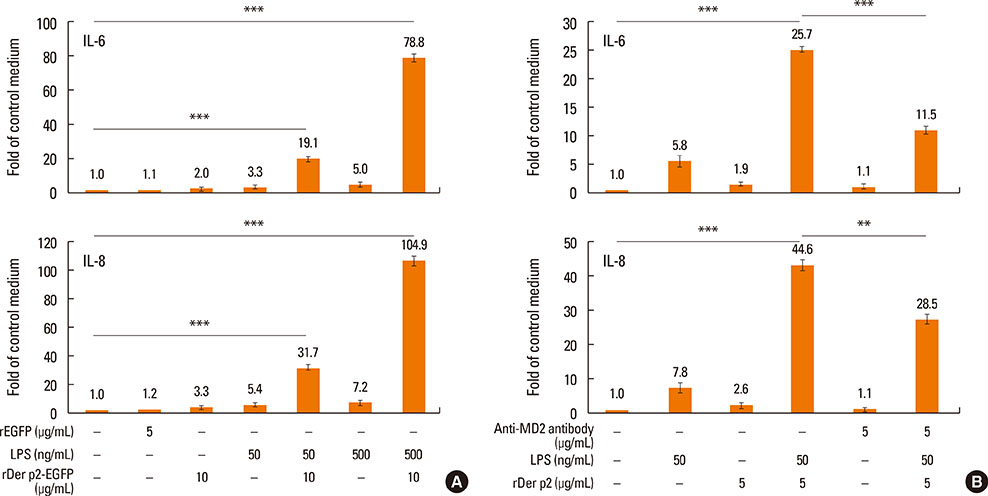
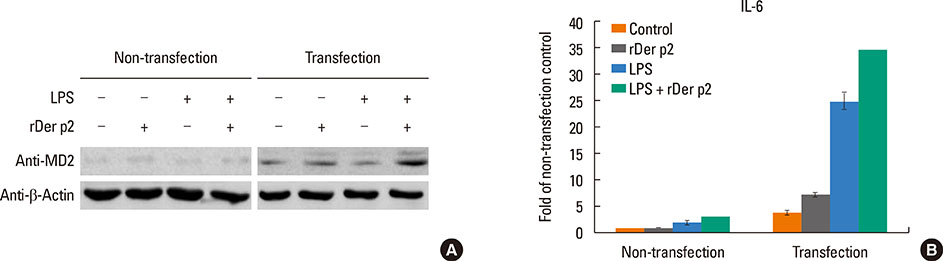
Internalization of recombinant, enhanced green fluorescent protein-labelled Der p2 (rDer p2-EGFP) into human airway epithelium (BEAS-2B) was tracked by laser confocal microscopy and confirmed by immunoblotting. Reverse-transcription polymerase chain reaction (RT-PCR), immunoblotting, and immunohistochemical staining were used to determine the effect of Der p2 on MD2 expression in vitro and ex vivo. Expression of messenger RNA (mRNA) encoding receptors/cytokines was measured by RT-PCR. Secretion of interleukin-6/interleukin-8 (IL-6/IL-8) was measured by enzyme-linked immunosorbent assay (ELISA).
RESULTS
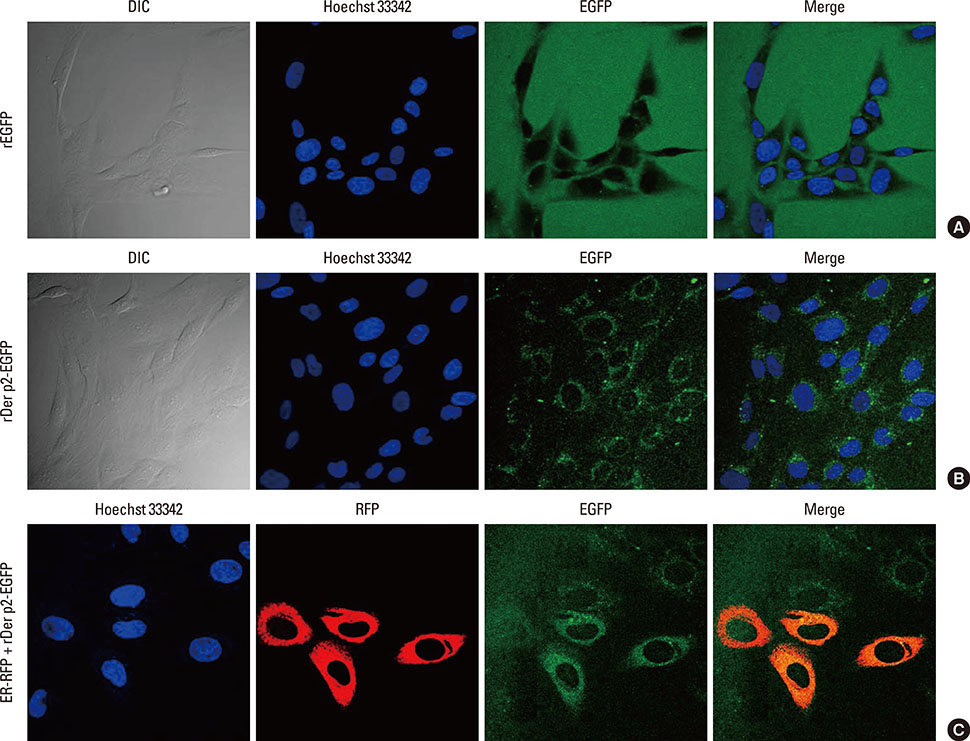
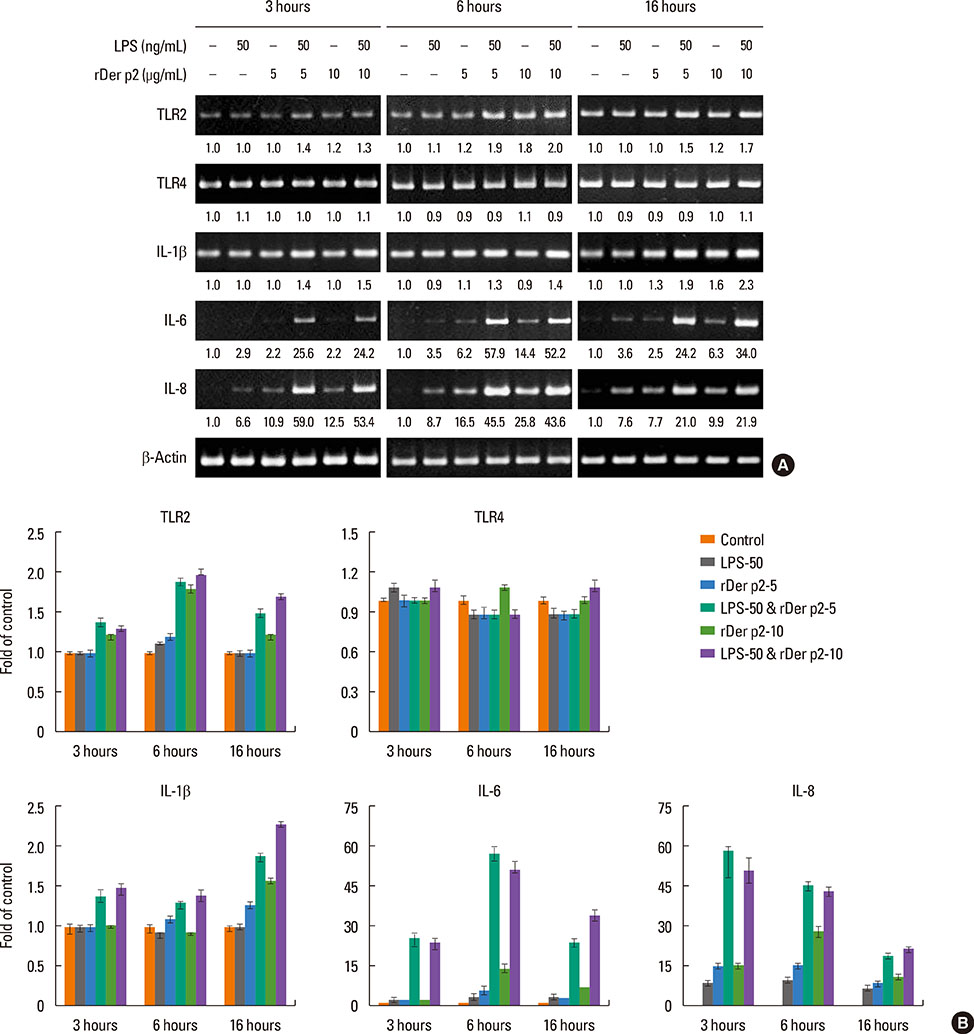
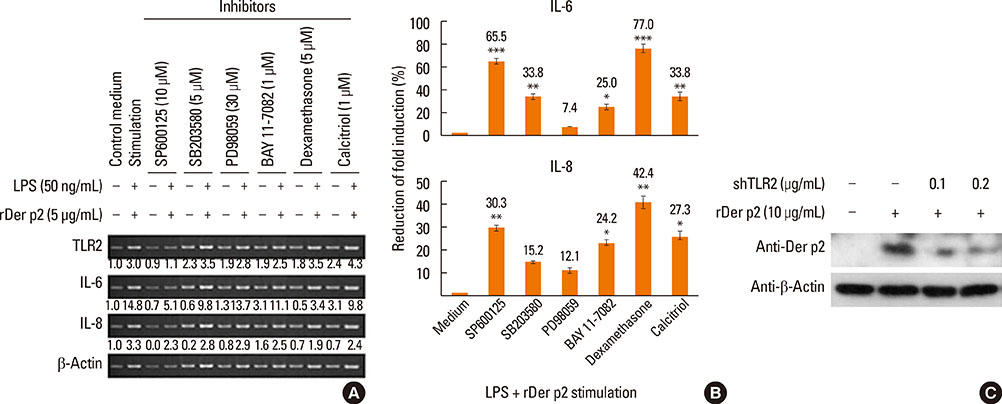
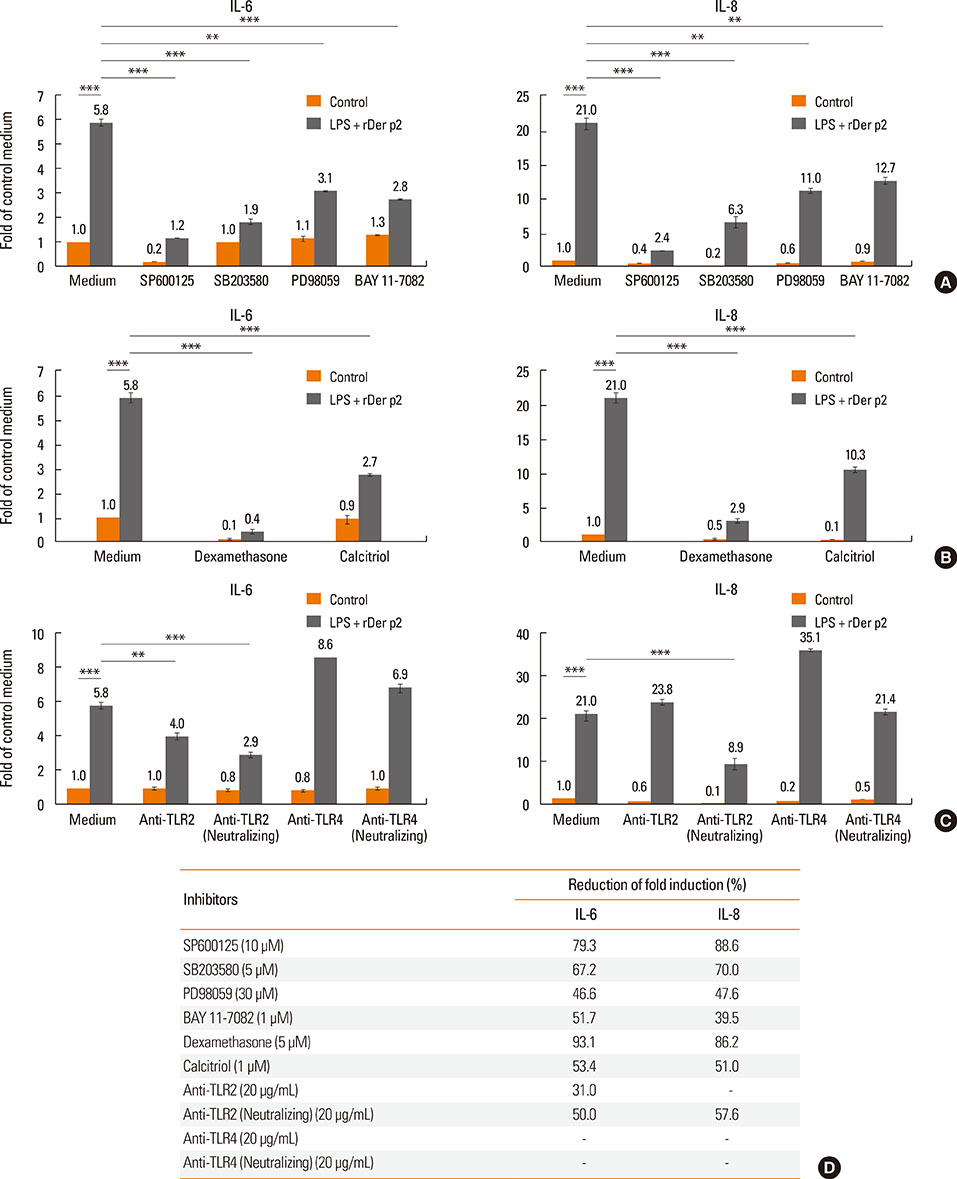
Internalization of Der p2 by BEAS-2B was confirmed by confocal microscopy and immunoblotting using rDer p2-EGFP and rDer p2, respectively. Expression of MD2 protein was increased in BEAS-2B and human nasal polyp airway epithelium cultured with rDer p2. Recombinant Der p2-cultured BEAS-2B caused little spontaneous IL-6/IL-8 secretion but significantly augmented by TLR ligand LPS. IL-6 secretion was up-regulated after MD2 transfection. Internalization of Der p2 was reduced by TLR2 RNA knockdown. Dexamethasone, calcitriol, anti-MD2/anti-TLR2 antibodies, and signalling inhibitors significantly reduced LPS+Der p2-induced IL-6/IL-8 secretion.
CONCLUSIONS
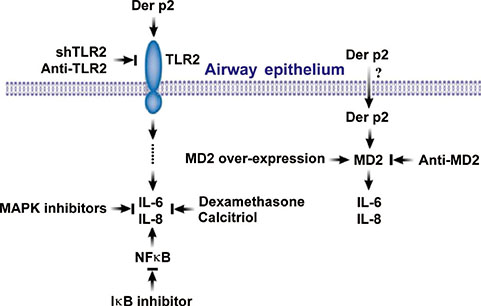
Human airway epithelium may internalize Der p2, which potentiates the response to environmental proinflammatory stimuli through MD2 and TLRs. This study highlights a novel mechanism and alleviates IL-6/IL-8 secretion in mite-induced airway inflammation.
Keyword
MeSH Terms
-
Antibodies
Calcitriol
Dexamethasone
Enzyme-Linked Immunosorbent Assay
Epithelium*
Humans
Immunoblotting
Inflammation
Interleukin-6
Microscopy, Confocal
Nasal Polyps
Polymerase Chain Reaction
RNA
RNA, Messenger
Toll-Like Receptor 2
Toll-Like Receptors
Transfection
Antibodies
Calcitriol
Dexamethasone
Interleukin-6
RNA
RNA, Messenger
Toll-Like Receptor 2
Toll-Like Receptors
Figure
Cited by 1 articles
-
The Role of Lipids in Development of Allergic Responses
Manuel Gómez del Moral, Eduardo Martínez-Naves
Immune Netw. 2017;17(3):133-143. doi: 10.4110/in.2017.17.3.133.
Reference
-
1. Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997; 100:S2–24.2. Tsai JJ, Kao MH, Huang SL. Comparison of major aeroallergens in Taipei and Kin-Men. J Formos Med Assoc. 1997; 96:985–989.3. Tsai JJ, Shen HD, Chua KY. Purification of group 2 Dermatophagoides pteronyssinus allergen and prevalence of its specific IgE in asthmatics. Int Arch Allergy Immunol. 2000; 121:205–210.4. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.5. Chiou YL, Lin CY. Der p2 activates airway smooth muscle cells in a TLR2/MyD88-dependent manner to induce an inflammatory response. J Cell Physiol. 2009; 220:311–318.6. Hsu SC, Chen CH, Tsai SH, Kawasaki H, Hung CH, Chu YT, et al. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), on human dendritic cells. J Biol Chem. 2010; 285:7903–7910.7. Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L428–L437.8. Tsai JJ, Liu SH, Yin SC, Yang CN, Hsu HS, Chen WB, et al. Mite allergen Der-p2 triggers human B lymphocyte activation and Toll-like receptor-4 induction. PLoS One. 2011; 6:e23249.9. Liu YH, Kao MC, Lai YL, Tsai JJ. Efficacy of local nasal immunotherapy for Dp2-induced airway inflammation in mice: using Dp2 peptide and fungal immunomodulatory peptide. J Allergy Clin Immunol. 2003; 112:301–310.10. Liao EC, Tsai JJ. Clinical effectiveness of Tyrophagus putrescentiae allergy by local nasal immunotherapy using strips of Dermatophagoides pteronyssinus. J Asthma. 2011; 48:957–964.11. Merkle D, Zheng D, Ohrt T, Crell K, Schwille P. Cellular dynamics of Ku: characterization and purification of Ku-eGFP. Chembiochem. 2008; 9:1251–1259.12. Rostkowska-Nadolska B, Latocha M, Gawron W, Kutner A, Bochnia M. The influence of calcitriol and tacalcitol on proliferation of fibroblasts cultured from nasal polyps. Adv Clin Exp Med. 2007; 16:213–219.13. Sato H, Ogino Y, Takagi H, Hata J, Asano S, Ohta T, et al. Pharmacological profiles of high-concentration (20 microg/g) tacalcitol ointment: effects on cutaneous inflammation, epidermal proliferation, and differentiation in mice. J Dermatol. 2003; 30:510–524.14. Tukaj S, Trzonkowski P, Tukaj C. Regulatory effects of 1,25-dihydroxyvitamin D3 on vascular smooth muscle cells. Acta Biochim Pol. 2012; 59:395–400.15. Clements D, Asprey SL, McCulloch TA, Morris TA, Watson SA, Johnson SR. Analysis of the oestrogen response in an angiomyolipoma derived xenograft model. Endocr Relat Cancer. 2009; 16:59–72.16. Giaid A, Michel RP, Stewart DJ, Sheppard M, Corrin B, Hamid Q. Expression of endothelin-1 in lungs of patients with cryptogenic fibrosing alveolitis. Lancet. 1993; 341:1550–1554.17. Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999; 274:10689–10692.18. Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999; 274:33419–33425.19. Wu TT, Chen TL, Loon WS, Tai YT, Cherng YG, Chen RM. Lipopolysaccharide stimulates syntheses of toll-like receptor 2 and surfactant protein-A in human alveolar epithelial A549 cells through upregulating phosphorylation of MEK1 and ERK1/2 and sequential activation of NF-kappaB. Cytokine. 2011; 55:40–47.20. Tanyaratsrisakul S, Jirapongsananuruk O, Thomas WR, Piboonpocanun S, Voelker DR. Der p2 stimulate inflammatory responses from lung epithelial cells and macrophages through the TLR2 and MAPK pathway. J Allergy Clin Immunol. 2012; 129:AB140.21. Xue ML, Zhu H, Thakur A, Willcox M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002; 80:340–345.22. Rostkowska-Nadolska B, Sliupkas-Dyrda E, Potyka J, Kusmierz D, Fraczek M, Krecicki T, et al. Vitamin D derivatives: calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv Med Sci. 2010; 55:86–92.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nucleic Acid Recognition and Signaling by Toll-like Receptor 9: Compartment-dependent Regulation
- Peptidoglycan Induces the Production of Interleukin-8 via Calcium Signaling in Human Gingival Epithelium
- IGF-I Exerts an Anti-inflammatory Effect on Skeletal Muscle Cells through Down-regulation of TLR4 Signaling
- Toll-like Receptors and Innate Immunity
- Enhanced Allergic Inflammation of Der p 2 Affected by Polymorphisms of MD-2 Promoter