J Korean Diabetes Assoc.
2007 May;31(3):200-207. 10.4093/jkda.2007.31.3.200.
The Effect of Alpha-lipoic Acid on the Cell Cycle Arrest and Apoptosis in Rat Vascular Smooth Muscle Cells
- Affiliations
-
- 1Department of Internal Medicine, Kyungpook National University, School of Medicine, Korea.
- 2Department of Microbiology, Kyungpook National University, College of Science and Technology, Korea.
- 3Division of Molecular and Life Sciences, College of Science and Technology, Hanyang University, Korea.
- 4Department of Internal Medicine, Keimyung University School of Medicine, Korea.
- KMID: 1523008
- DOI: http://doi.org/10.4093/jkda.2007.31.3.200
Abstract
-
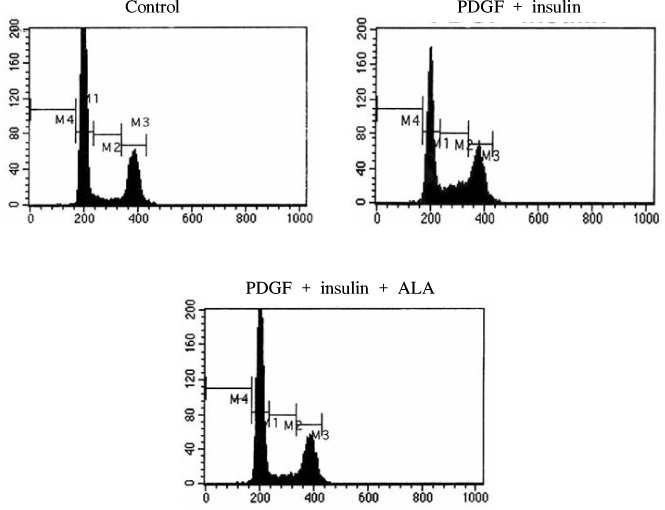
BACKGROUND: The proliferation of vascular smooth muscle cells (VSMCs) is a hallmark of atheroscelrosis and post-angioplasty restenosis. We previously showed that alpha-lipoic acid (ALA) inhibited neointimal hyperplasia and has potential anti-atherosclerosis effect in rat carotid artery balloon injured model. Here, we investigated whether alpha-lipoic acid inhibited proliferation of cells and induced apoptosis in rat vascular smooth muscle cells.
METHODS
VSMCs were treated with ALA under each condition, harvested and protein was extracted. Same amount of protein was loaded into SDS-PAGE and western blot analysis was performed with various cell cycle regulation protein. To examine ALA induce apoptosis in VSMCs, FACS and DNA fragmentation assay were performed. Antioxidant effect of ALA was determined by DCF-DA staining.
RESULTS
ALA induced VSMCs cell cycle arrest and induced p21, p27 and p53 proteins. Also ALA induced PTEN expression and AMPK phosphorylation. Increased AMPK phosphorylation reduced Erk-2 phosphorylation and finally arrested cell cycle promotion. The apoptotic effect was also shown by ALA treatment. Also we confirmed that ALA reduced ROS generation in VSMCs.
CONCLUSION
The present data suggest that ALA has anti-proliferative effect and arrests cell proliferation. Therefore, ALA may provide new strategies for the prevention of neointimal hyperplasia after angioplasty.
MeSH Terms
-
AMP-Activated Protein Kinases
Angioplasty
Animals
Antioxidants
Apoptosis*
Blotting, Western
Carotid Arteries
Cell Cycle Checkpoints*
Cell Cycle*
Cell Proliferation
DNA Fragmentation
Electrophoresis, Polyacrylamide Gel
Hyperplasia
Muscle, Smooth, Vascular*
Phosphorylation
Rats*
Thioctic Acid*
AMP-Activated Protein Kinases
Antioxidants
Thioctic Acid
Figure
Reference
-
1. Mensah GA, Mokdad AH, Ford E, Narayan KM, Giles WH, Vinicor F, Deedwania PC. Obesity, metabolic syndrome, and type 2 diabetes: emerging epidemics and their cardiovascular implications. Cardiol Clin. 2004. 22:485–504.2. Liu MW, Roubin GS, King SB III. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989. 79:1374–1387.3. Ardissino D, Di Somma S, Kubica J, Barberis P, Merlini PA, Eleuteri E, De Servi S, Bramucci E, Specchia G, Montemartini C. Influence of elastic recoil on restenosis after successful coronary angioplasty in unstable angina pectoris. Am J Cardiol. 1993. 71:659–663.4. Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999. 85:753–766.5. Barks JL, McQuillan JJ, Iademarco MF. TNF-alpha and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol. 1997. 159:4532–4538.6. Ross R. The pathogenesis of atherosclerosis; a perspective for the 1990s. Nature. 1993. 362:801–809.8. Bhoday J, De Silva S, Xu Q. The molecular mechanism of vascular restenosis Which genes are crucial? Curr Vasc Pharmacol. 2006. 4:269–275.9. Durand E, Mallat Z, Addad F, Vilde F, Desnos M, Guent C, Tedqui A, Lafont A. Time course of apoptosis and cell proliferation and their relationship to arterial remodeling and restenosis after angioplasty in an atherosclerotic rabbit model. J Am Coll Cardiol. 2002. 39:1680–1685.10. O'Sullivana M, Scotta SD, McCarthya N, Figga N, Shapirob LM, Kirkpatrickc P, Bennetta MR. Differential cyclin E expression in human in-stent stenosis smooth muscle cells identifies targets for selective anti-restenosis therapy. Cardiovascular Res. 2003. 60:673–683.11. Raymond MA, Désormeaux A, Laplante P, Vigneault N, Filep JG, Landry K, Pshezhetsky AV, Hébert MJH. Apoptosis of endothelial cells triggers a caspase-dependent anti-apoptotic paracrine loop active on vascular smooth muscle cells. FASEB J. 2004. 18:705–707.12. Biewnga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacy. 1997. 3:315–331.13. Lester Pr, Eric H, Hans JT. Alpha lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995. 19:227–250.14. Kim MS, Park JY, Namkoomg C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha JH, Park IS, Lee IK, Benoit V, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nature medicine. 2004. 10:727–733.15. Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo IH, Oh GT, Par IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005. 25:2488–2494.16. Yi X, Maeda N. Alpha lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low cholesterol diet. Diabetes. 2006. 55:2238–2244.17. Lee KM, Park KG, Kim YD, Lee HJ, Kim HT, Cho WH, Kim HS, Han SW, Koh GY, Park JY, Lee KU, Kim JG, Lee IK. Alpha-lipoic acid inhibits fractalkine expression and prevents neointimal hyperplasia after balloon injury in rat carotid artery. Atherosclerosis. 2006. 189:106–113.18. Karyn M, James C, Kosta S, Susan P, Douglas F. Alpha-lipoic acid induces p27Kip-dependent cell cycle arrest in non-transformed cell lines and apoptosis in tumor cell lines. J Cell Physiol. 2003. 194:325–340.19. Simbula G, Columbano A, Ledda-Columbano GM, Sanna L, Deidda M, Diana A, Pibiri M. Increased ROS generation and p53 activation in alpha-lipoic acid-induced apoptosis of hepatoma cells. Apoptosis. 2007. 12:113–123.20. Ahn JD, Morishita R, Kaneda Y, Lee SJ, Kwon KY, Choi SY, Lee KU, Park JY, Moon IJ, Park JG, Yoshizumi M, Ouchi Y, Lee IK. Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ Res. 2002. 90:1325–1332.22. Kim HJ, Park KG, Yoo EK, Kim YH, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim BW, Lee IK. Effects of PGC-1alpha on TNF-alpha-Induced MCP-1 and VCAM-1 Expression and NF-kappaB Activation in Human Aortic Smooth Muscle and Endothelial Cells. Antioxid Redox Signal. 2007. in press.24. Targonsky ED, Dai F, Koshkin V, Karaman GT, Gyulkhandanyan AV, Zhang Y, Chan CB, Wheeler MB. Alpha-lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Diabetologia. 2006. 49:1587–1598.25. Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrionology. TRENDS Endocrionl Metab. 2006. 17:205–215.26. Tokumitsu W, Masao Y, Masahiro A, Masato E, Kenji T, Masayoshi H, Koichiro N, Yi-Qiang L, Yumiko O, Katsuya I, Noriko S, Kim SB, Takashi N, Naohide Y, Junya A, Yasuyoshi O. Induction of nuclear orphan receptor NGFI-B gene and apoptosis in rat vascular smooth muscle cells treated with pyrrolidinedithiocarbamate. Arterioscler Thromb Vasc Biol. 2001. 21:1738–1744.27. Stephen W, Stephen B. Induction of apoptosis in fetal pulmonary arterial smooth muscle cells by a combined superoxide dismutase/catalase mimetic. Am J Physiol Lung Cell Mol Physiol. 2003. 285:L305–L312.28. Mizuno M, Packer L. Effects of α-Lipoic Acid and Dihydrolipoic Acid on Expression of Proto-oncogene c-fos. Biochem Biophys Res Commun. 1994. 200:1136–1142.29. Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, Kerum G, Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid. Diabetes Care. 1999. 22:1296–1301.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiproliferative Effect of Lovastatin on Vascular Smooth Muscle Cell
- The Effect of alpha-Lipoic Acid on Vascular Smooth Muscle Cell Proliferation, Migration, Neointimal Formation and PAI-1 Expression
- Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells
- Alpha-Lipoic acid Inhibits TNF-alpha-Induced Fractalkine Expression in Rat aortic Smooth Muscle Cells
- Effects of alpha-Lipoic Acid on Apoptotic Cell Death in Rat Hippocampus Following Transient Forebrain Ischemia-reperfusion Injury