Nutr Res Pract.
2009 Dec;3(4):265-271.
Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells
- Affiliations
-
- 1Department Food Science and Nutrition, Dankook University, 126 Jukjeon-dong, Suji-gu, Yongin-si, Gyunggi 448-701, Korea. wkkim@dankook.ac.kr
Abstract
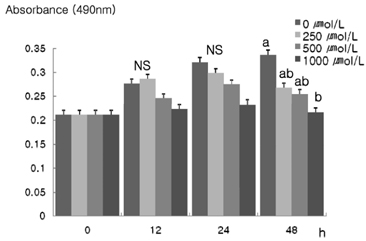
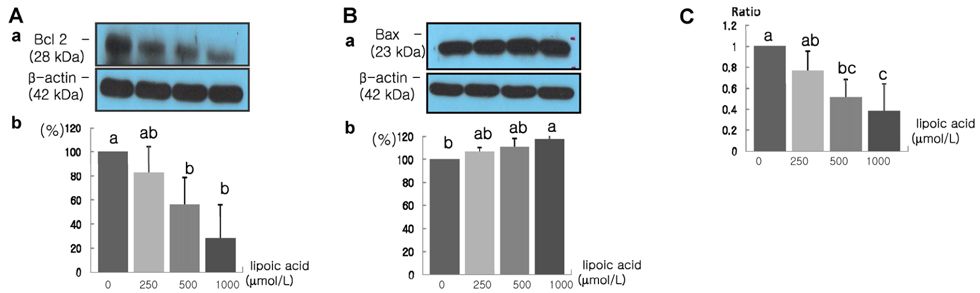
- The role that antioxidants play in the process of carcinogenesis has recently gained considerable attention. alpha-Lipoic acid, a naturally occurring disulfide molecule, is a powerful antioxidant that reportedly exerts beneficial effects in patients with advanced cancer by reducing the level of reactive oxygen species and increasing glutathione peroxidase activity. In this study, we examined changes in the protein and mRNA expression associated with cell proliferation and apoptosis in MDA-MB-231 breast cancer cultured in the presence of various concentrations (0, 250, 500, and 1000 micromol/L) of alpha-lipoic acid. The results revealed that alpha-lipoic acid inhibited the growth of breast cancer cells in a dose-independent manner (P < 0.05). Additionally, ErbB2 and ErbB3 protein and mRNA expressions were significantly decreased in a dose-dependent manner in response to alpha-lipoic acid (P < 0.05). Furthermore, the protein expression of phosphorylated Akt (p-Akt) levels and total Akt, and the mRNA expression of Akt were decreased dose-dependently in cells that were treated with alpha-lipoic acid (P < 0.05). Bcl-2 protein and mRNA expressions were also decreased in cells that were treated with alpha-lipoic acid (P < 0.05). However, Bax protein and mRNA expressions were increased in cells treated with alpha-lipoic acid (P < 0.05). Finally, caspase-3 activity was significantly increased in a dose-dependent manner in cells treated with alpha-lipoic acid (P < 0.05). In conclusion, we demonstrated that alpha-lipoic acid inhibits cell proliferation and induces apoptosis in MDA-MB-231 breast cancer cell lines.
MeSH Terms
-
Antioxidants
Apoptosis
bcl-2-Associated X Protein
Breast
Breast Neoplasms
Caspase 3
Cell Line
Cell Proliferation
Glutathione Peroxidase
Humans
Reactive Oxygen Species
RNA, Messenger
Thioctic Acid
Antioxidants
Caspase 3
Glutathione Peroxidase
RNA, Messenger
Reactive Oxygen Species
Thioctic Acid
bcl-2-Associated X Protein
Figure
Reference
-
1. Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997. 64:33–42.
Article2. Bianco R, Gelardi T, Damiano V, Ciardiello F, Tortora G. Rational bases for the development of EGFR inhibitors for cancer treatment. Int J Biochem Cell Biol. 2007. 39:1416–1431.
Article3. Cameron NE, Coteer MA, Horrobin DH, Tritschler HJ. Effects of alpha-lipoic acid on neurovascular function in diabetic rats: interation with essential fatty acids. Diabetologia. 1998. 41:390–399.
Article4. Gurer H, Ozgunes H, Oztezcan S, Ercal N. Antioxidant role of alpha-lipoic acid in lead toxicity. Free Radic Biol Med. 1999. 27:75–81.5. Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A. 1994. 91:8132–8136.
Article6. Hortbagyi GN, de la Garza Salazar J, Pritchard K, Amodori D, Haidinger R, Hudis CA, Khaled H, Liu MC, Martin M, Namer M, O'Shaughnessy JA, Shen ZZ, Albain KS. The global breast cancer burden:variation in epidemiology and survival. Clin Breast Cancer. 2005. 6:391–401.7. Huang WY, Newman B, Milliken RC, Conway K, Hulka BS, Schell MJ, Liu ET. Risk of breast cancer according to the status of HER-2/neu oncogene amplification. Cancer Epidemiol Biomarkers Prev. 2000. 9:65–71.8. Jeoung SY. The effect of lipoic acid on antioxidant enzyme system in murine melanoma cells. 2006. Seoul. Republic of Korea: SookMyung University;Master thesis.9. Kang HJ, Kim SW, Yun YK, Oh SK, Choe KJ, Noh DY. Expression of p53, c-erbB2, bcl-2, Cathepsin D in lnfiltrating Ductal Cancer of the Breast. Jornal of the Korean Surgical Society. 2001. 60:592–599.10. Kim WK, Bang MH, Kim ES, Kang NE, Jung KC, Cho HJ, Park JHY. Quercetin decreases the expression of ErB2 and ErB3 proteins in HT-29 human colon cancer cells. J Nutr Biochem. 2005. 16:155–162.
Article11. Larghero P, Vene R, Minghelli S, Travaini G, Morini M, Ferrari N, Pfeffer U, Noonan DM, Albini A, Benelli R. Biological assays and genomic analysis reveal lipoic acid modulation of endothelial cell behavior and gene expression. Carcinogenesis. 2007. 28:1008–1020.
Article12. Lee HS, Seo EY, Kim WK. Resveratrol induces apoptosis in SW480 human colon cancer cell lines. Food Sci Biotechnol. 2004. 13:80–84.13. Marsh SA, Laursen PB, Pat BK, Gobe GC, Coombes JS. Bcl-2 in endothelial cells is increased by vitamin E and alpha-lipoic acid supplementation but not exercise training. J Mol Cell Cardiol. 2005. 38:445–451.
Article14. Annual report of National Cancer Registration. Ministry of Health and Welfare and Family. 2008. 8/14/2009. https://u-lib.nanet.go.kr/dl/SimpleView.php.15. Moungjaroen J, Nimmannit U, Callery PS, Wang L, Azad N, Lipipun V, Chanvorachote P, Rojanasakul Y. Reactive oxygen species mediate caspase activation and apoptosis induced by lipoic acid on human lung epithelial cancer cell through Bcl-2 down regulation. J Pharmacol Exp Ther. 2006. 319:1062–1069.
Article16. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000. 19:3159–3167.
Article17. Pack RA, Hardy K, Madigan MC, Hunt NH. Differential effects of the antioxidant alpha-lipoic acid on the proliferation of mitogenstimulated peripheral blood lymphocytes and leukemic T cell. Mol Immunol. 2002. 38:733–745.
Article18. Packer L. alpha-Lipoic acid: a metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab Rev. 1998. 30:245–275.
Article19. Palacios J, Robles-Frias MJ, Castilla MA, Lopez-Garcia MA, Benitez J. The molecular pathology of hereditary breast cancer. Pathobiology. 2008. 75:85–94.
Article20. Perez-Nadales E, Lloyd AC. Essential function for ErbB3 in breast cancer proliferation. Breast Cancer Res. 2004. 6:137–139.
Article21. Reed LJ, Debusk BG, Cunsalus IC, Hornberger CS Jr. Crystalline alpha-lipoic acid; a catalytic agent associated with pyruvate dehydrogenase. Science. 1951. 27:93–94.22. Riese DJ, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998. 20:41–48.
Article23. Sen CK, Sashwati R, Packer L. Fas mediated apoptosis of human Jurkat T-cells: intracellular events and potentiation by redox-active alpha-lipoic acid. Cell Death Differ. 1999. 6:481–491.
Article24. Seo EY, Kim WK. Effect of [6]-gingerol on bcle-2 and Bax expression in MDA-MB-231 human breast cancer cell line. Journal of the Korean Society of Food Science and Nutrition. 2006. 35:671–676.
Article25. Simbula G, Columbano A, Ledda-Columbano GM, Sanna L, Deidda M, Diana A, Pibiri M. Increased ROS generation and p53 activation in alpha-lipoic acid -induced apoptosis of hepatoma cells. Apoptosis. 2007. 12:113–123.
Article26. Vig-Varga E, Benson EA, Limbil TL, Allison BM, Geobl MG, Harrington MA. Alpha-lipoic acid modulate ovarian surface epithelial cell growth. Gynecol Oncol. 2006. 103:45–52.27. Wenzel U, Nickel A, Daniel H. alpha-Lipoic acid induces apoptosis in human colon cancer cells by increasing mitochondrial respiration with a concomitant O2-*- generation. Apoptosis. 2005. 10:359–368.
Article28. Xue C, Liang F, Mahmood R, Vuolo M, Wyckoff J, Qian H, Tsai KL, Kim MM, Locker J, Zhang ZY, Segall JE. ErbB3-Dependent Motility and Intravasation in Breast Cancer Metastasis. Cancer Res. 2006. 66:1418–1426.
Article29. Zhai H, Chen X, Hu Z. Study on the relationship between intake of trace elements and breast cancer mortality with chemometric methods. Comput Biol Chem. 2003. 27:581–586.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of corosolic acid on apoptosis and angiogenesis in MDA-MB-231 human breast cancer cells
- Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells
- Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines
- Comparative Studies on the Polyarnine Involvement in MCF - 7 and MDA - MB - 231 Breast Cancer Cell Proliferation
- The anti-cancer effect of pomegranate-derived nanovesicles on MDA-MB-231 breast cancer cells