Anat Cell Biol.
2011 Sep;44(3):210-217. 10.5115/acb.2011.44.3.210.
Modulation by the GABAB receptor siRNA of ethanol-mediated PKA-alpha, CaMKII, and p-CREB intracellular signaling in prenatal rat hippocampal neurons
- Affiliations
-
- 1Department of Biology, College of Natural Sciences (RINS), Gyeongsang National University, Jinju, Korea. mokim@gnu.ac.kr
- 2National Institute of Animal Science, Suwon, Korea.
- 3Department of Agronomy, Research Institute of Life Science, College of Veterinary Medicine, Gyeongsang National University, Jinju, Korea.
- 4Department of Anatomy, Research Institute of Life Science, College of Veterinary Medicine, Gyeongsang National University, Jinju, Korea.
- KMID: 1447433
- DOI: http://doi.org/10.5115/acb.2011.44.3.210
Abstract
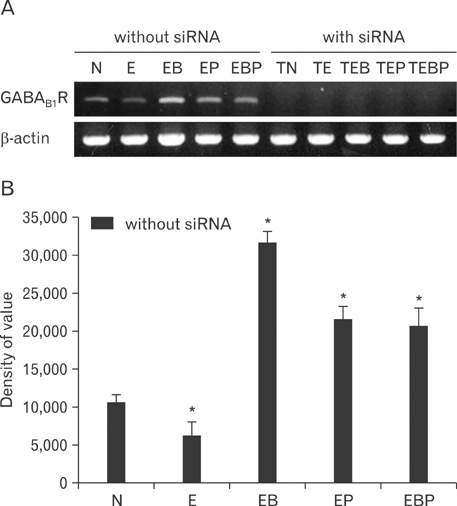
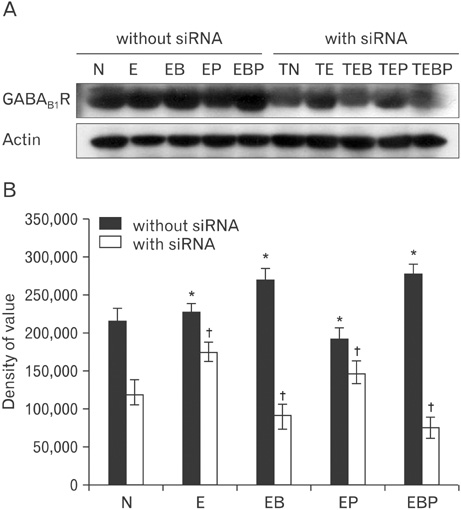
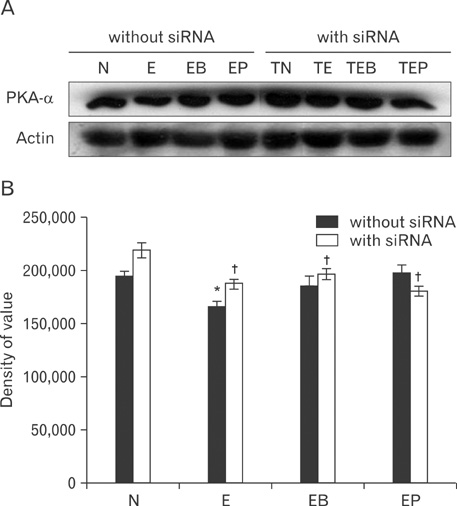
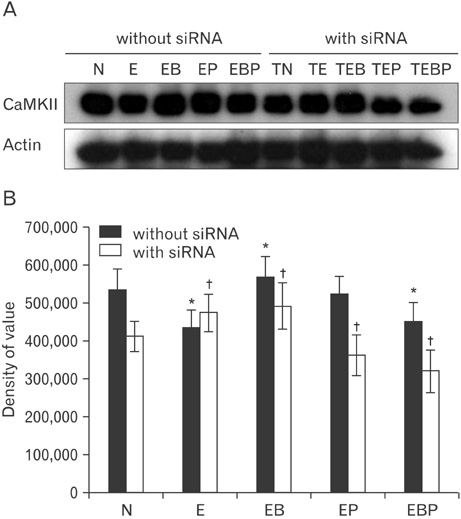
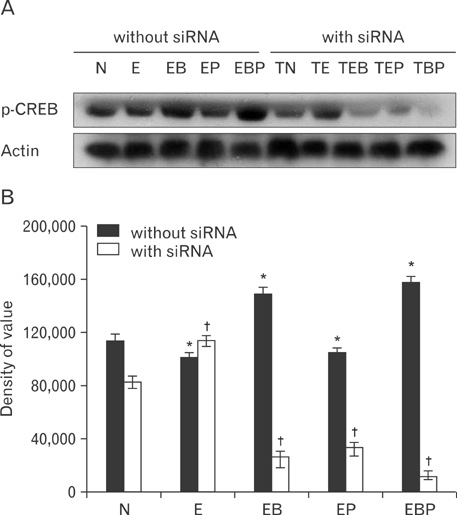
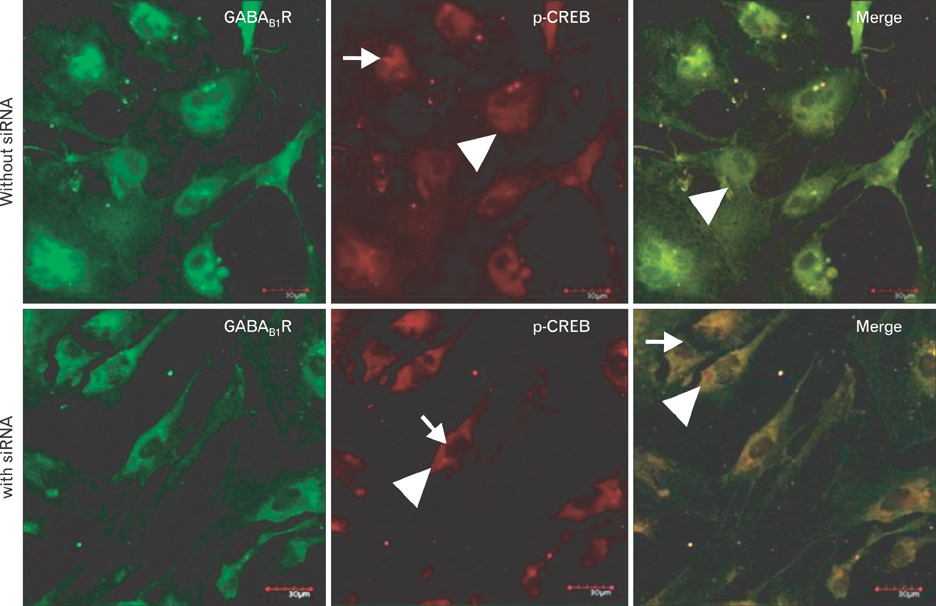
- Fetal alcohol syndrome (FAS) is a developmental neuropathology resulting from in utero exposure to ethanol; many of ethanol's effects are likely to be mediated by the neurotransmitter gamma-aminobutyric acid (GABA). We studied modulation of the neurotransmitter receptor GABABR and its capacity for intracellular signal transduction under conditions of ethanol treatment (ET) and RNA interference to investigate a potential role for GABA signaling in FAS. ET increased GABAB1R protein levels, but decreased protein kinase A-alpha (PKA-alpha), calcium/calmodulin-dependent protein kinase II (CaMKII) and phosphorylation of cAMP-response element binding protein (p-CREB), in cultured hippocampal neurons harvested at gestation day 17.5. To elucidate GABAB1R response to ethanol, we observed the effects of a GABABR agonist and antagonist in pharmacotherapy for ethanol abuse. Baclofen increased GABABR, CaMKII and p-CREB levels, whereas phaclofen decreased GABABR, CaMKII and p-CREB levels except PKA-alpha. Furthermore, when GABAB1R was knocked down by siRNA treatment, CaMKII and p-CREB levels were reduced upon ET. We speculate that stimulation of GABAB1R activity by ET can modulate CaMKII and p-CREB signaling to detrimental effect on fetal brain development.
Keyword
MeSH Terms
-
Animals
Baclofen
Brain
Calcium-Calmodulin-Dependent Protein Kinase Type 2
Carrier Proteins
Ethanol
Fetal Alcohol Syndrome
gamma-Aminobutyric Acid
Hippocampus
Neurons
Neurotransmitter Agents
Phosphorylation
Pregnancy
Protein Kinases
Rats
Receptors, Neurotransmitter
RNA Interference
RNA, Small Interfering
Signal Transduction
Baclofen
Calcium-Calmodulin-Dependent Protein Kinase Type 2
Carrier Proteins
Ethanol
Neurotransmitter Agents
Protein Kinases
RNA, Small Interfering
Receptors, Neurotransmitter
gamma-Aminobutyric Acid
Figure
Reference
-
1. Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med. 1978. 298:1063–1067.2. Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000. 10:94–110.3. Lindsley TA, Comstock LL, Rising LJ. Morphologic and neurotoxic effects of ethanol vary with timing of exposure in vitro. Alcohol. 2002. 28:197–203.4. Byrnes ML, Richardson DP, Brien JF, Reynolds JN, Dringenberg HC. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt: prenatal ethanol exposure. Neurotoxicol Teratol. 2004. 26:543–551.5. Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973. 302:999–1001.6. Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991. 265:1961–1967.7. Miller MW, Nowakowski RS. Effect of prenatal exposure to ethanol on the cell cycle kinetics and growth fraction in the proliferative zones of fetal rat cerebral cortex. Alcohol Clin Exp Res. 1991. 15:229–232.8. Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001. 25:175–184.9. Ticku MK, Lowrimore P, Lehoullier P. Ethanol enhances GABA-induced 36Cl-influx in primary spinal cord cultured neurons. Brain Res Bull. 1986. 17:123–126.10. Toso L, Roberson R, Woodard J, Abebe D, Spong CY. Prenatal alcohol exposure alters GABA(A)alpha5 expression: a mechanism of alcohol-induced learning dysfunction. Am J Obstet Gynecol. 2006. 195:522–527.11. Allan AM, Harris RA. A new alcohol antagonist: phaclofen. Life Sci. 1989. 45:1771–1779.12. Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002. 54:247–264.13. Duthey B, Caudron S, Perroy J, Bettler B, Fagni L, Pin JP, Prézeau L. A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J Biol Chem. 2002. 277:3236–3241.14. Besheer J, Lepoutre V, Hodge CW. GABA(B) receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology (Berl). 2004. 174:358–366.15. Enna SJ, Bowery NG. GABA(B) receptor alterations as indicators of physiological and pharmacological function. Biochem Pharmacol. 2004. 68:1541–1548.16. Escher T, Mittleman G. Effects of ethanol and GABAB drugs on working memory in C57BL/6J and DBA/2J mice. Psychopharmacology (Berl). 2004. 176:166–174.17. Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav. 2004. 78:743–750.18. Li SP, Park MS, Jin GZ, Kim JH, Lee HL, Lee YL, Kim JH, Bahk JY, Park TJ, Koh PO, Chung BC, Kim MO. Ethanol modulates GABA(B) receptor expression in cortex and hippocampus of the adult rat brain. Brain Res. 2005. 1061:27–35.19. Dzitoyeva S, Dimitrijevic N, Manev H. Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc Natl Acad Sci U S A. 2003. 100:5485–5490.20. Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005. 115:2762–2773.21. Li SP, Kim JH, Park MS, Bahk JY, Chung BC, Kim MO. Ethanol modulates the expression of GABA(B) receptor mRNAs in the prenatal rat brain in an age and area dependent manner. Neuroscience. 2005. 134:857–866.22. Bison S, Crews F. Alcohol withdrawal increases neuropeptide Y immunoreactivity in rat brain. Alcohol Clin Exp Res. 2003. 27:1173–1183.23. Peris J, Eppler B, Hu M, Walker DW, Hunter BE, Mason K, Anderson KJ. Effects of chronic ethanol exposure on GABA receptors and GABAB receptor modulation of 3H-GABA release in the hippocampus. Alcohol Clin Exp Res. 1997. 21:1047–1052.24. Wang Z, Hu SY, Lei DL, Song WX. Effect of chronic stress on PKA and P-CREB expression in hippocampus of rats and the antagonism of antidepressors. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006. 31:767–771.25. Asyyed A, Storm D, Diamond I. Ethanol activates cAMP response element-mediated gene expression in select regions of the mouse brain. Brain Res. 2006. 1106:63–71.26. Pandey SC, Chartoff EH, Carlezon WA Jr, Zou J, Zhang H, Kreibich AS, Blendy JA, Crews FT. CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcohol Clin Exp Res. 2005. 29:176–184.27. Dave KR, Lange-Asschenfeldt C, Raval AP, Prado R, Busto R, Saul I, Pérez-Pinzón MA. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J Neurosci Res. 2005. 82:665–673.28. Franěk M. History and the present of metabotropic GABAB receptor. Cesk Fysiol. 2004. 53:117–124.29. Kamatchi GL, Ticku MK. Functional coupling of presynaptic GABAB receptors with voltage-gated Ca2+ channel: regulation by protein kinases A and C in cultured spinal cord neurons. Mol Pharmacol. 1990. 38:342–347.30. Knight AR, Bowery NG. The pharmacology of adenylyl cyclase modulation by GABAB receptors in rat brain slices. Neuropharmacology. 1996. 35:703–712.31. Kubota H, Katsurabayashi S, Moorhouse AJ, Murakami N, Koga H, Akaike N. GABAB receptor transduction mechanisms, and cross-talk between protein kinases A and C, in GABAergic terminals synapsing onto neurons of the rat nucleus basalis of Meynert. J Physiol. 2003. 551(Pt 1):263–276.32. Mukherjee RS, McBride EW, Beinborn M, Dunlap K, Kopin AS. Point mutations in either subunit of the GABAB receptor confer constitutive activity to the heterodimer. Mol Pharmacol. 2006. 70:1406–1413.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perampanel ameliorates nitroglycerin-induced migraine through inhibition of the cAMP/ PKA/CREB signaling pathway in the trigeminal ganglion in rats
- Phasic and Tonic Inhibition are Maintained Respectively by CaMKII and PKA in the Rat Visual Cortex
- Effects of Apigenin on Glutamate-induced [Ca2+]i Increases in Cultured Rat Hippocampal Neurons
- Diarylpropionitrile inhibits melanogenesis via protein kinase A/cAMP-response element-binding protein/microphthalmiaassociated transcription factor signaling pathway in α-MSHstimulated B16F10 melanoma cells
- The Blocking of TNF-alpha by RNA Interference and Its Influence on Synovial Fibroblast and Chondrocytes