Korean J Physiol Pharmacol.
2014 Dec;18(6):517-524. 10.4196/kjpp.2014.18.6.517.
Phasic and Tonic Inhibition are Maintained Respectively by CaMKII and PKA in the Rat Visual Cortex
- Affiliations
-
- 1Department of Physiology, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. hjjang@catholic.ac.kr
- 2Catholic Neuroscience Institute, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2285555
- DOI: http://doi.org/10.4196/kjpp.2014.18.6.517
Abstract
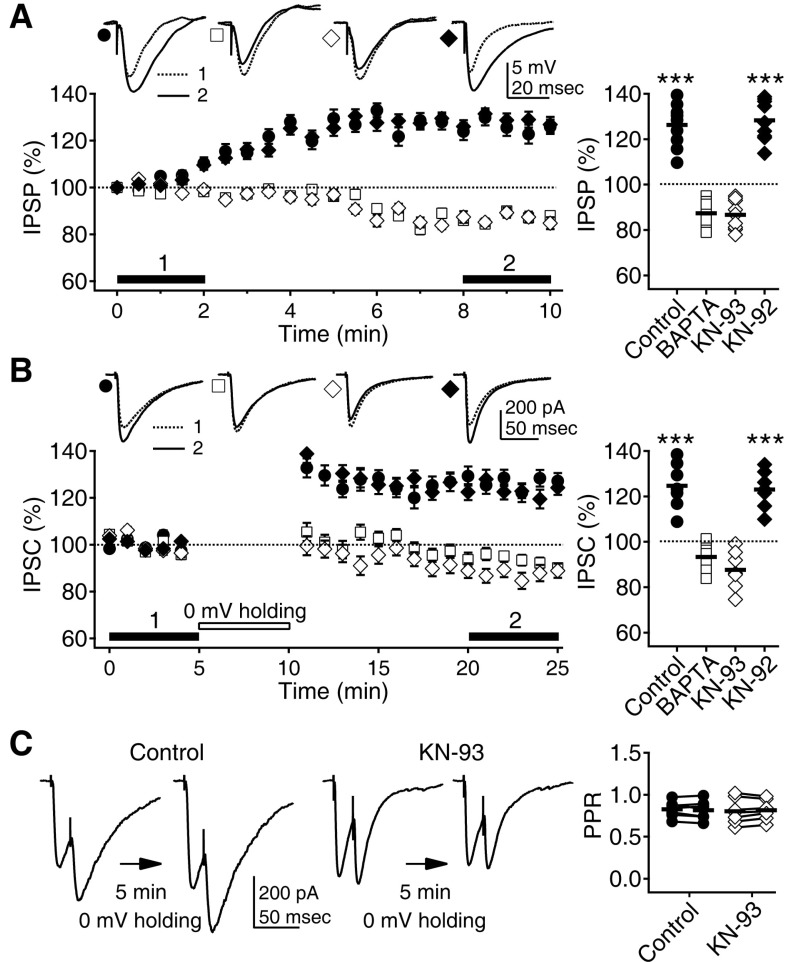
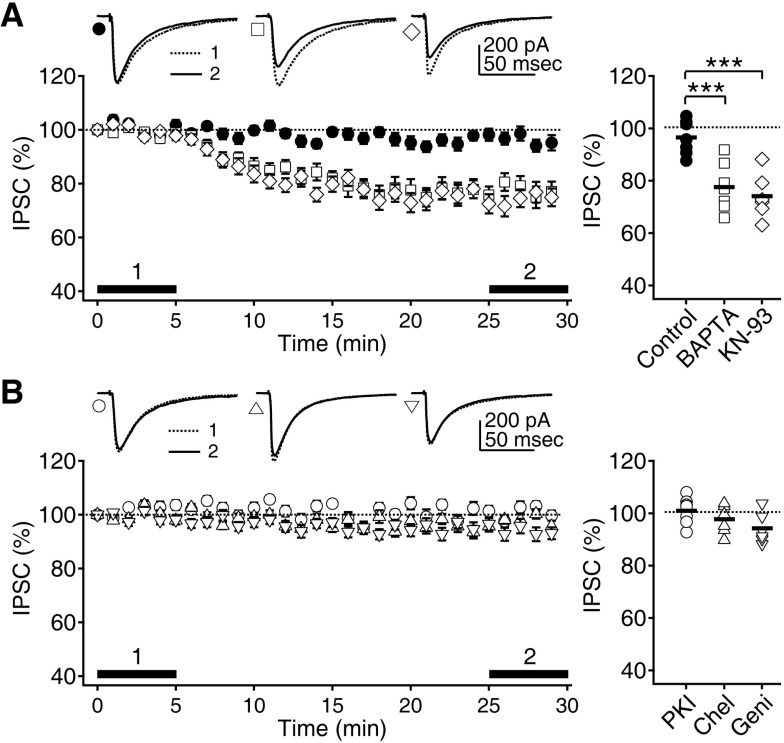
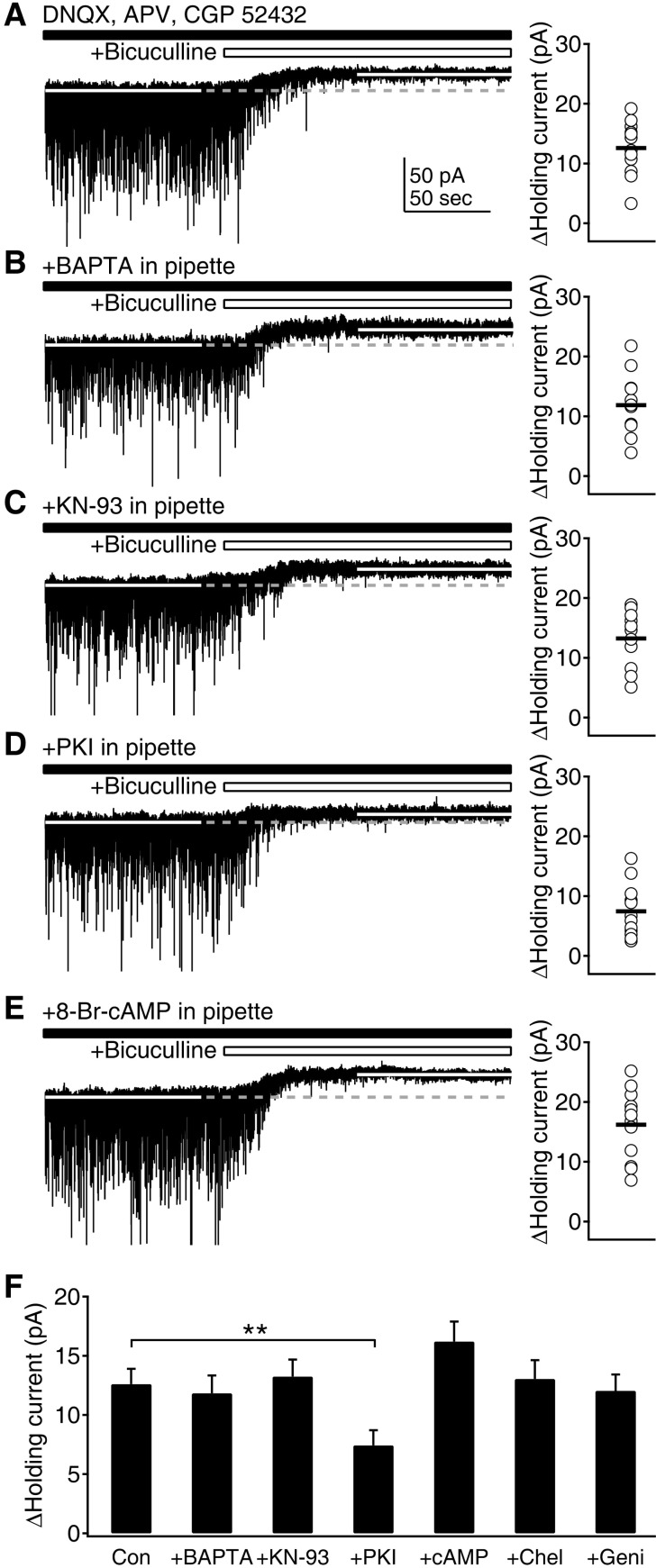
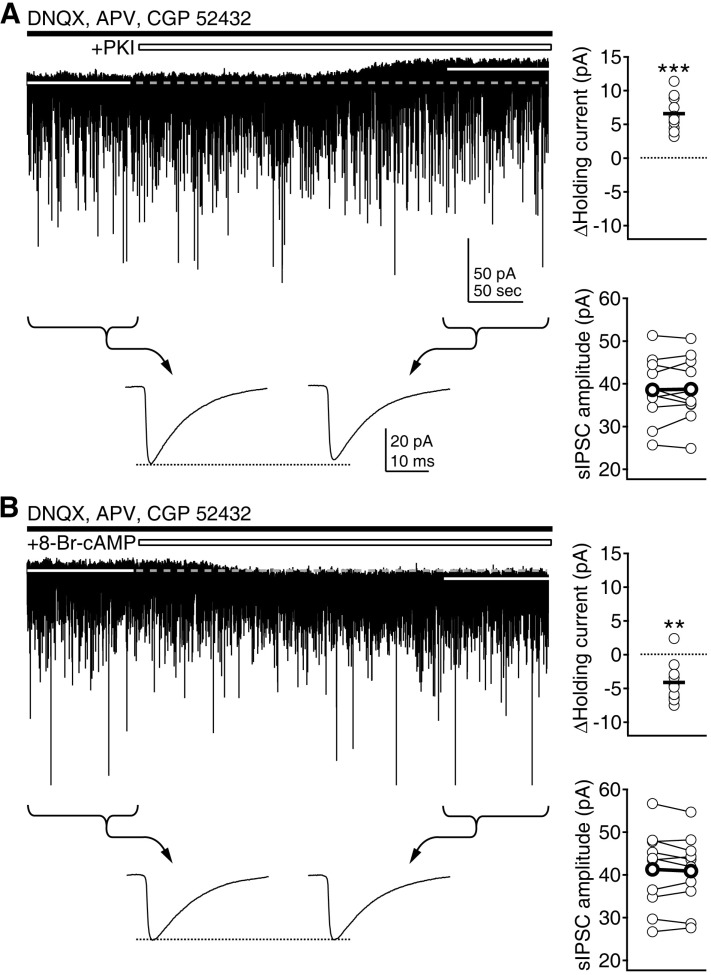
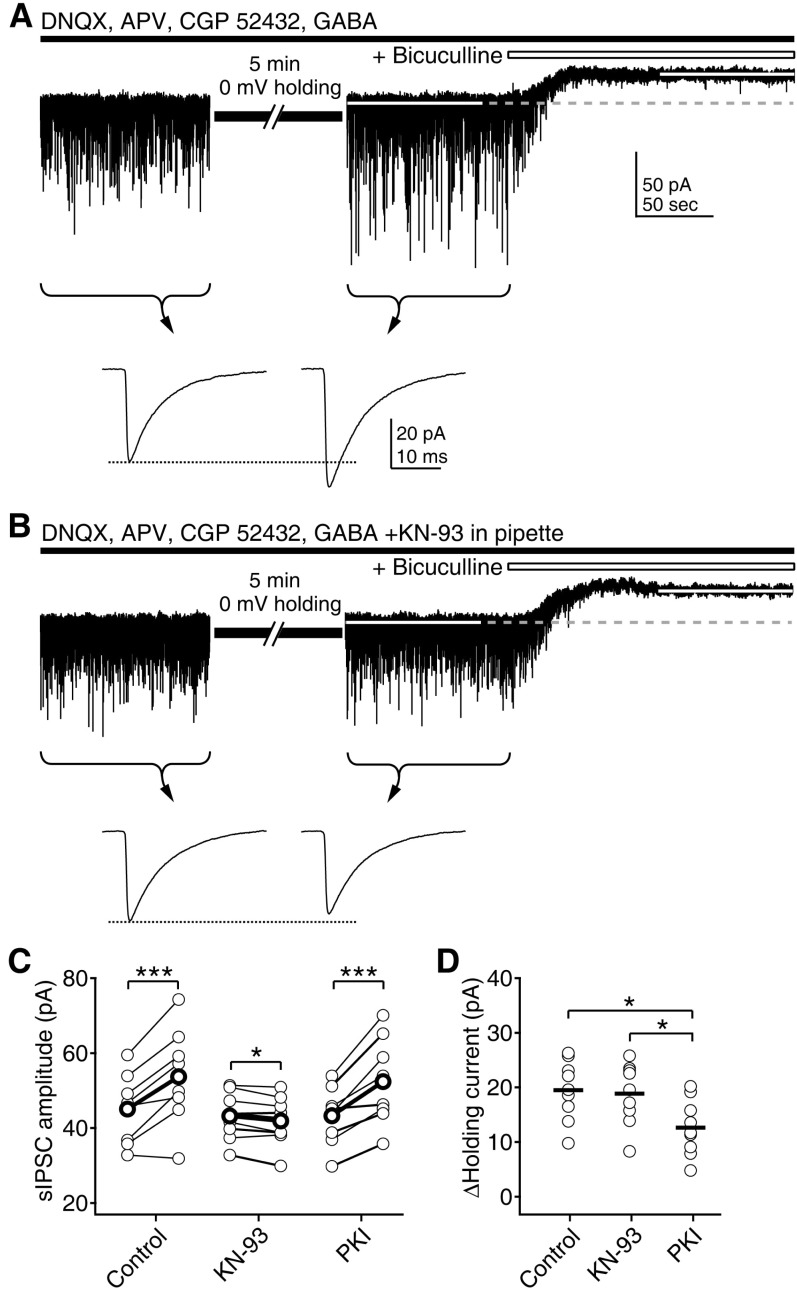
- Phasic and tonic gamma-aminobutyric acid(A) (GABA(A)) receptor-mediated inhibition critically regulate neuronal information processing. As these two inhibitory modalities have distinctive features in their receptor composition, subcellular localization of receptors, and the timing of receptor activation, it has been thought that they might exert distinct roles, if not completely separable, in the regulation of neuronal function. Inhibition should be maintained and regulated depending on changes in network activity, since maintenance of excitation-inhibition balance is essential for proper functioning of the nervous system. In the present study, we investigated how phasic and tonic inhibition are maintained and regulated by different signaling cascades. Inhibitory postsynaptic currents were measured as either electrically evoked events or spontaneous events to investigate regulation of phasic inhibition in layer 2/3 pyramidal neurons of the rat visual cortex. Tonic inhibition was assessed as changes in holding currents by the application of the GABA(A) receptor blocker bicuculline. Basal tone of phasic inhibition was maintained by intracellular Ca2+ and Ca2+/calmodulin-dependent protein kinase II (CaMKII). However, maintenance of tonic inhibition relied on protein kinase A activity. Depolarization of membrane potential (5 min of 0 mV holding) potentiated phasic inhibition via Ca2+ and CaMKII but tonic inhibition was not affected. Thus, phasic and tonic inhibition seem to be independently maintained and regulated by different signaling cascades in the same cell. These results suggest that neuromodulatory signals might differentially regulate phasic and tonic inhibition in response to changes in brain states.
MeSH Terms
-
Animals
Automatic Data Processing
Bicuculline
Brain
Calcium-Calmodulin-Dependent Protein Kinase Type 2*
Cyclic AMP-Dependent Protein Kinases
Inhibitory Postsynaptic Potentials
Membrane Potentials
Nervous System
Neurons
Protein Kinases
Rats*
Receptors, GABA-A
Visual Cortex*
Bicuculline
Calcium-Calmodulin-Dependent Protein Kinase Type 2
Cyclic AMP-Dependent Protein Kinases
Protein Kinases
Receptors, GABA-A
Figure
Cited by 1 articles
-
Enhancement of GluN2B Subunit-Containing NMDA Receptor Underlies Serotonergic Regulation of Long-Term Potentiation after Critical Period in the Rat Visual Cortex
Kayoung Joo, Duck-Joo Rhie, Hyun-Jong Jang
Korean J Physiol Pharmacol. 2015;19(6):523-531. doi: 10.4196/kjpp.2015.19.6.523.
Reference
-
1. Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron. 2012; 75:951–962. PMID: 22998865.
Article2. Fritschy JM, Panzanelli P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur J Neurosci. 2014; 39:1845–1865. PMID: 24628861.3. Möhler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006; 326:505–516. PMID: 16937111.
Article4. Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005; 6:215–229. PMID: 15738957.
Article5. Frégnac Y, Monier C, Chavane F, Baudot P, Graham L. Shunting inhibition, a silent step in visual cortical computation. J Physiol Paris. 2003; 97:441–451. PMID: 15242656.
Article6. Livingstone MS. Directional inhibition: a new slant on an old question. Neuron. 2005; 45:5–7. PMID: 15629696.7. Jang HJ, Cho KH, Kim HS, Hahn SJ, Kim MS, Rhie DJ. Age-dependent decline in supragranular long-term synaptic plasticity by increased inhibition during the critical period in the rat primary visual cortex. J Neurophysiol. 2009; 101:269–275. PMID: 18971296.
Article8. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Effects of Serotonin on the Induction of Long-term Depression in the Rat Visual Cortex. Korean J Physiol Pharmacol. 2010; 14:337–343. PMID: 21165334.
Article9. Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000; 404:183–186. PMID: 10724170.
Article10. Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003; 13:341–347. PMID: 12850219.
Article11. Connelly WM, Errington AC, Di Giovanni G, Crunelli V. Metabotropic regulation of extrasynaptic GABAA receptors. Front Neural Circuits. 2013; 7:171. PMID: 24298239.
Article12. Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002; 22:RC223. PMID: 12006605.
Article13. Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001; 26:907–913. PMID: 11699942.14. Jang HJ, Cho KH, Kim MJ, Yoon SH, Rhie DJ. Layer- and cell-type-specific tonic GABAergic inhibition of pyramidal neurons in the rat visual cortex. Pflugers Arch. 2013; 465:1797–1810. PMID: 23812164.
Article15. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. The development of phasic and tonic inhibition in the rat visual cortex. Korean J Physiol Pharmacol. 2010; 14:399–405. PMID: 21311681.
Article16. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Layer-specific serotonergic facilitation of IPSC in layer 2/3 pyramidal neurons of the visual cortex. J Neurophysiol. 2012; 107:407–416. PMID: 22013240.
Article17. Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000; 101:815–850. PMID: 11113332.
Article18. Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009; 29:12757–12763. PMID: 19828786.
Article19. Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003; 100:14439–14444. PMID: 14623958.20. Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012; 73:23–34. PMID: 22243744.
Article21. Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT. Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol Rev. 2011; 63:243–267. PMID: 21245208.
Article22. Hirano T, Kawaguchi SY. Regulation and functional roles of rebound potentiation at cerebellar stellate cell-Purkinje cell synapses. Front Cell Neurosci. 2014; 8:42. PMID: 24600347.
Article23. Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br J Pharmacol. 2004; 142:9–19. PMID: 15100161.
Article24. Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011; 477:171–178. PMID: 21796121.
Article25. Vogels TP, Abbott LF. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat Neurosci. 2009; 12:483–491. PMID: 19305402.
Article26. Zhang Z, Sun QQ. The balance between excitation and inhibition and functional sensory processing in the somatosensory cortex. Int Rev Neurobiol. 2011; 97:305–333. PMID: 21708316.
Article27. Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010; 465:932–936. PMID: 20559387.
Article28. Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007; 30:119–125. PMID: 17267052.
Article29. Whitt JL, Petrus E, Lee HK. Experience-dependent homeostatic synaptic plasticity in neocortex. Neuropharmacology. 2014; 78:45–54. PMID: 23466332.
Article30. Gogolla N, Leblanc JJ, Quast KB, Sdhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009; 1:172–181. PMID: 20664807.
Article31. Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci. 2008; 1:5. PMID: 18946538.32. Eichler SA, Meier JC. E-I balance and human diseases - from molecules to networking. Front Mol Neurosci. 2008; 1:2. PMID: 18946535.
Article33. Vogels TP, Sprekeler H, Zenke F, Clopath C, Gerstner W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science. 2011; 334:1569–1573. PMID: 22075724.
Article34. Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005; 147:115–124. PMID: 15581701.
Article35. Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010; 20:456–467. PMID: 19520765.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Development of Phasic and Tonic Inhibition in the Rat Visual Cortex
- The Effects of Partial Outlet Obstruction on Bladder Strip Sensitivity to Glucose Deprivation: An In-Vitro Study in the Rat
- Role of Nitric Oxide in the Motor Activity of Rat Vas Deferens
- Effects of Relief of Bladder Outlet Obstruction on the Detrusor Contractility in Rat
- Changes of beta-Adrenergic Receptor mRNA in the Visual Cortex and Superior Colliculus of Monocular Deprivated Rat