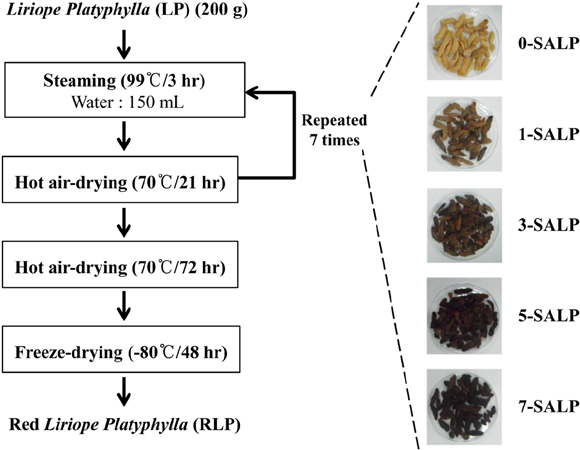
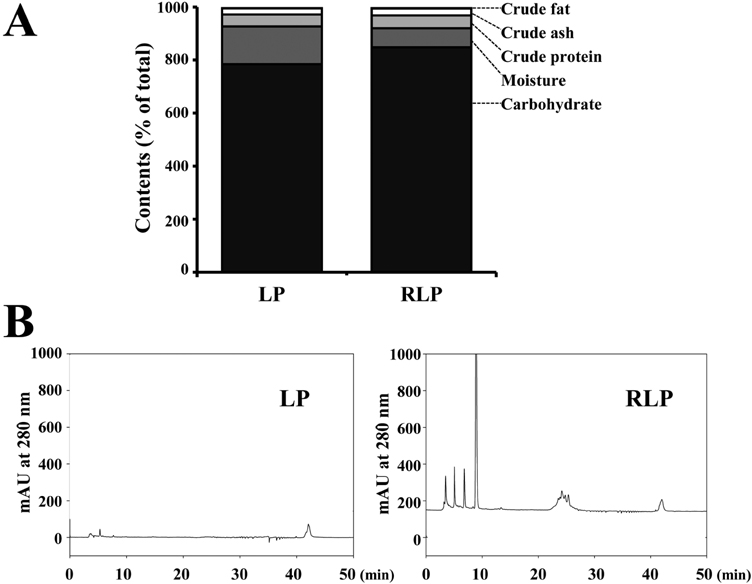
Lab Anim Res.
2012 Dec;28(4):229-238. 10.5625/lar.2012.28.4.229.
Toxicity of red Liriope platyphylla manufactured by steaming process on liver and kidney organs of ICR mice
- Affiliations
-
- 1College of Natural Resources & Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2College of Human Ecology, Pusan National University, Busan, Korea.
- 3Sandong Nonghub, Miryang, Korea.
- 4Department of Experimental Animal Research, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- KMID: 1431405
- DOI: http://doi.org/10.5625/lar.2012.28.4.229
Abstract
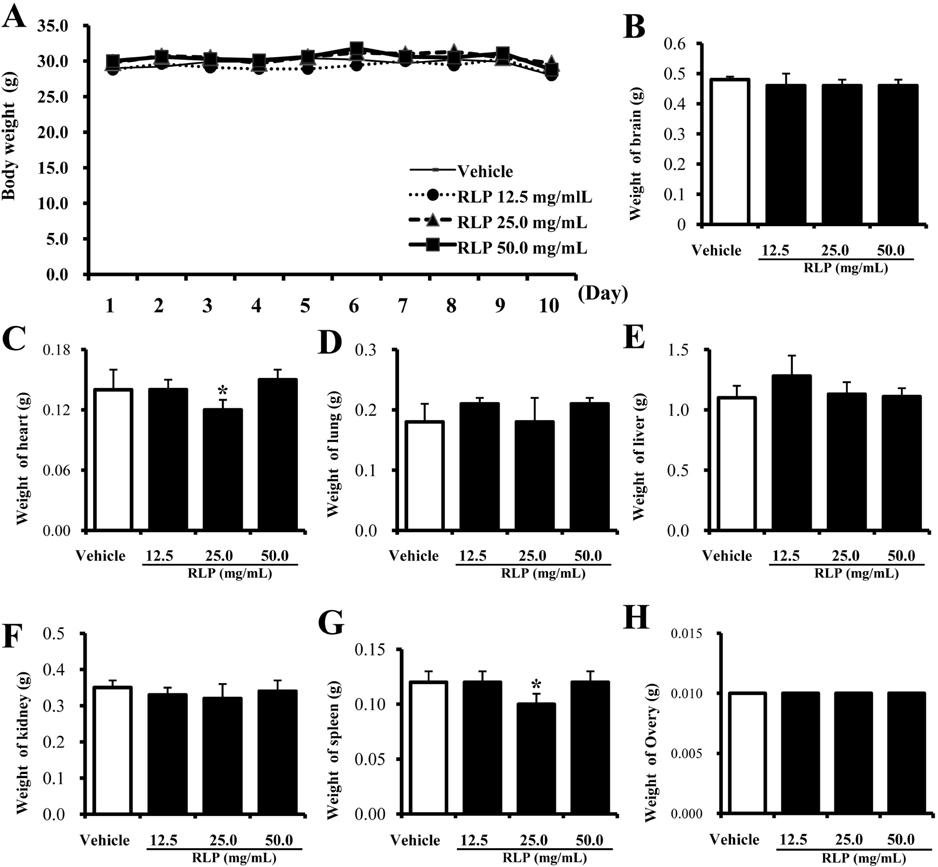
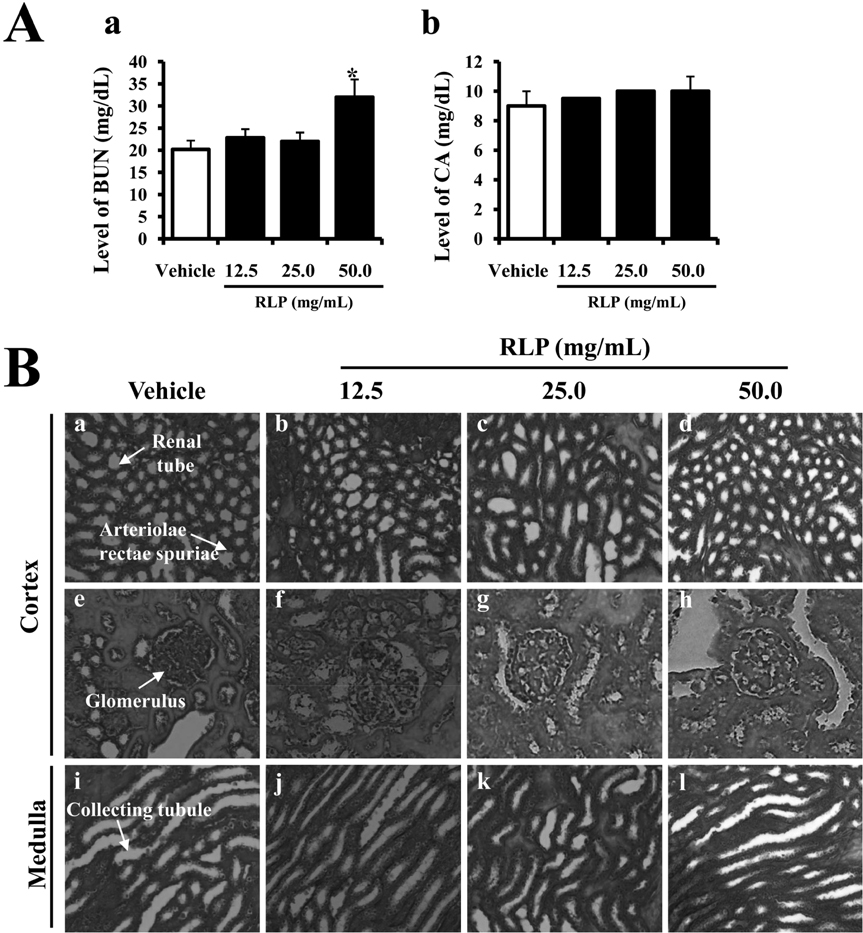
- Red Liriope platyphylla (RLP) produced by steaming process has been reported to enhance the secretion of insulin and nerve growth factor (NGF). However, there has been no report on the toxicity of RLP in the specific organs of mice. To investigate the toxic effect of RLP, we tried to observe a significant alteration on body weight, food/water intake, organ weight, liver pathology and kidney pathology in female ICR mice received 12.5, 25.0 and 50.0 mg/kg body weight/day of RLP via gavage for 10 days. Out of seven organs including brain, heart, lung, liver, kidney, spleen and ovary, two organs (heart and lung) showed significantly decreased weights in the medium dosage RLP-treated group, whereas weights of other organs were maintained at constant levels in all dosage groups. In the liver toxicity analysis, no significant increase of alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate amino-transferase (AST) were detected in any RLP-treated group compared to vehicle-treated group. The specific pathological changes induced by most of toxic compounds were not observed in the liver in microscopic examination. Furthermore, in the kidney toxicological analysis, a significant enhancement of the blood urea nitrogen (BUN) concentration was detected in the high dosage RLP-treated group compared to the vehicle-treated group. However, the serum creatinine (CA) concentration on the serum biochemistry as well as the pathological changes in microscopic examination were not significantly different between the vehicle- and RLP-treated groups. Therefore, these results suggest that RLP does not induce any specific toxicity in liver or kidney tissues of mice, although the BUN level slightly increased in 50.0 mg/kg of RLP-treated group.
Keyword
MeSH Terms
-
Alanine Transaminase
Alkaline Phosphatase
Animals
Aspartic Acid
Biochemistry
Blood Urea Nitrogen
Body Weight
Brain
Creatinine
Female
Heart
Humans
Insulin
Kidney
Liver
Lung
Mice
Mice, Inbred ICR
Nerve Growth Factor
Organ Size
Ovary
Spleen
Steam
Weights and Measures
Alanine Transaminase
Alkaline Phosphatase
Aspartic Acid
Creatinine
Insulin
Nerve Growth Factor
Steam
Figure
Reference
-
1. Kim ES, Choi SJ, Lee CW, Park KT, Moon JY. Protective effects of Liliaceae root extracts on amyloid β-protein-induced death in neuronal cells. Cancer Prev Res. 2005. 10:242–250.2. Huh MK, Huh HW, Choi JS, Lee BK. Genetic diversity and population structure of Liriope platyphylla (Liliaceae) in Korea. J Life Sci. 2007. 17:328–333.3. Choi SI, Lee HR, Goo JS, Kim JE, Nam SH, Hwang IS, Lee YJ, Prak SH, Lee HS, Lee JS, Jang IS, Son HJ, Hwang DY. Effects of Steaming Time and Frequency for Manufactured Red Liriope platyphylla on the Insulin Secretion Ability and Insulin Receptor Signaling Pathway. Lab Anim Res. 2011. 27(2):117–126.4. Kim JH, Kim JE, Lee YK, Nam SH, Her YK, Jee SW, Kim SG, Park DJ, Choi YW, Hwang DY. The extracts from Liriope platyphylla significantly stimulated insulin secretion in the HIT-T15 pancreatic β-cell line. J Life Sci. 2010. 20:969–1150.5. Lee YK, Kim JE, Nam SH, Goo JS, Choi SI, Choi YH, Bae CJ, Woo JM, Cho JS, Hwang DY. Differential regulation of the biosynthesis of glucose transporters by the PI3-K and MAPK pathways of insulin signaling by treatment with novel compounds from Liriope platyphylla. Int J Mol Med. 2011. 27(3):319–327.6. Kim JE, Nam SH, Choi SI, Hwang IS, Lee HR, Jang MJ, Lee CY, Soon HJ, Lee HS, Kim HS, Kang BC, Hong JT, Hwang DY. Aqueous extracts of Liriope platyphylla are tightly-regulated by insulin secretion from pancreatic islets and by increased glucose uptake through glucose transporters expressed in liver hepatocytes. Biomol Ther. 2011. 19:348–356.7. Choi SI, Park JH, Her YK, Lee YK, Kim JE, Nam SH, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. Effects of water extract of Liriope platyphylla on the mRNA expression and protein secretion of nerve growth factors. Korean J Med Crop Sci. 2010. 18:291–297.8. Hur J, Lee P, Kim J, Kim AJ, Kim H, Kim SY. Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biol Pharm Bull. 2004. 27(8):1257–1260.9. Hur J, Lee P, Moon E, Kang I, Kim SH, Oh MS, Kim SY. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol. 2009. 620(1-3):9–15.10. Nam SH, Choi SI, Goo JS, Kim JE, Lee YK, Hwang IS, Lee HR, Lee YJ, Lee HG, Choi YW, Hwang DY. LP-M, a Novel butanol-extracts isolated from Liriope platyphylla, could induce the neuronal cell survival and neuritic outgrowth in hippocampus of mice through Akt/ERK activation on NGF signal pathway. J Life Sci. 2011. 21:1234–1243.11. Kim JE, Lee YK, Nam SH, Choi SI, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. The symptoms of atopic dermatitis in NC/Nga mice were significantly relieved by the water extract of Liriope platyphylla. Lab Anim Res. 2010. 26:377–384.12. Weon JB, Ma JY, Yang HJ, Lee BH, Yun BR, Ma CJ. Enhancement of bioactivity of sosihotang with steaming process. Korean J Pharmacogn. 2011. 42:271–275.13. Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009. 7(3):293–302.14. Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng). J Pharm Pharmacol. 2006. 58(8):1007–1019.15. Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003. 68(8):1539–1542.16. Kim SD, Ku YS, Lee IZ, Kim ID, Youn KS. General components and sensory evaluation of hot water extract from Liriopis Tuber. J Korean Soc Food Sci Nutr. 2001. 30:20–24.17. Choi JH, Oh DH. Effect of white and red ginseng extracts on the immunological activities in lymphocytes isolated from sasang constitution blood Cells. J Ginseng Res. 2009. 33:33–39.18. Kong BM, Park MJ, Min JW, Kim HB, Kim SH, Kim SY, Yang DC. Physico-chemical characteristics of white, fermented and red ginseng extracts. J Ginseng Res. 2008. 32:238–243.19. Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996. 62(1):86–87.20. Nam KY. The comparative understanding between red ginseng and white ginsengs, processed ginsengs (panax ginseng C. A. Meyer). J Ginseng Res. 2005. 29:1–18.21. Sung H, Jung YS, Cho YK. Beneficial effects of a combination of Korean red ginseng and highly active antiretroviral therapy in human immunodeficiency virus type 1-infected patients. Clin Vaccine Immunol. 2009. 16(8):1127–1131.22. Yun TK, Lee YS, Kwon HY, Choi KJ. Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao. 1996. 17(4):293–298.23. Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007. 168(2):176–185.24. Ryu KS, Lee HS, Kim SY. Effects of Bombyx mori larvae extracts on carbon tetrachloride-induced hepatotoxicity in mice. J Life Sci. 1999. 9:375–381.25. Stripp B, Hamrick ME, Gillete JR. Effect of 3-methylcholanthrene induction on the carbon tetrachloride-induced changes in rat hepatic microsomal enzyme system. Biochem Pharmacol. 1972. 21(5):745–747.26. Recknigel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967. 19:145–208.27. You AS, Jeong MH, Park KH, Kim BS, Lee JB, Choi JH, Kwon OK, Kim JH. Effect on antioxidant function of onion to reduce pesticides toxicity. Korean J Pestic Sci. 2007. 11:222–229.28. Bergmeyer HU. Methods of enzymatic analysis. 1974. Vol 1. Weinheim: Verlag Chemie, Academic Press;20.29. Yoon SH, Park EJ, Oh KH, Chung YG, Kwon OJ. The effect of Lithospermi radix on benzo(a)pyrene - induced hepatotoxicity. J Korean Soc Food Nutr. 1993. 22:109–252.30. Hayes AW. Principles and methods of toxicology. 1982. New York: Raven Press;447–474.31. Kim DH, Deung YK, Lee YM, Yoon YS, Kwon KR, Park DB, Park YK, Lee KJ. The liver protecting effect of Pomegranate (Punica granatum) seed oil in mice treated with CCl4. Korean J Electron Microsc. 2006. 36:173–182.32. Horiguchi H, Oguma E, Kayama F, Sato M, Fukushima M. Dexamethasone prevents acute cadmium-induced hepatic injury but exacerbates kidney dysfunction in rabbits. Toxicol Appl Pharmacol. 2001. 174(3):225–234.33. Van De Graaff KM. Human anatomy. 2002. Sixth Edition. New York: McGraw-Hill;93–113.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Steaming Time and Frequency for Manufactured Red Liriope platyphylla on the Insulin Secretion Ability and Insulin Receptor Signaling Pathway
- Precautionary effects of Red Liriope platyphylla on NGF secretion and Abeta42 deposition under the preclinical stage of Alzheimer's disease in Tg2576 mice
- Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and gamma-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease
- Toxicity of fermented soybean product (cheonggukjang) manufactured by mixed culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on liver and kidney of ICR mice
- Beneficial effects of ethanol extracts of Red Liriope platyphylla on vascular dysfunction in the aorta of spontaneously hypertensive rats