Lab Anim Res.
2012 Sep;28(3):155-163. 10.5625/lar.2012.28.3.155.
Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and gamma-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease
- Affiliations
-
- 1College of Natural Resources & Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2College of Human Ecology, Pusan National University, Busan, Korea.
- 3Nonghyup Sandong Processing Plant, Miryang, Korea.
- KMID: 1436720
- DOI: http://doi.org/10.5625/lar.2012.28.3.155
Abstract
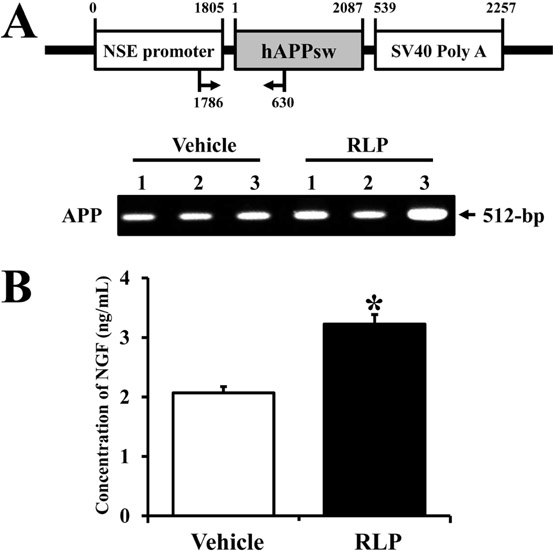
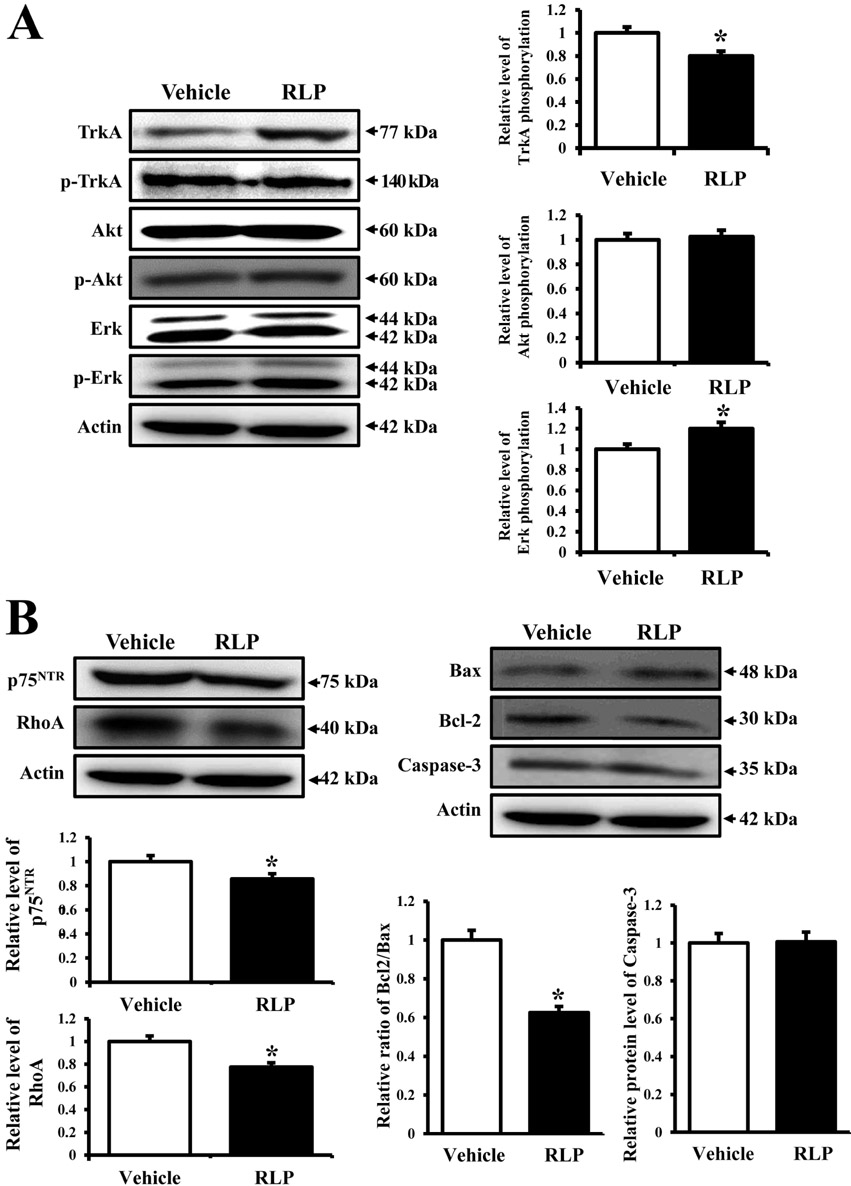
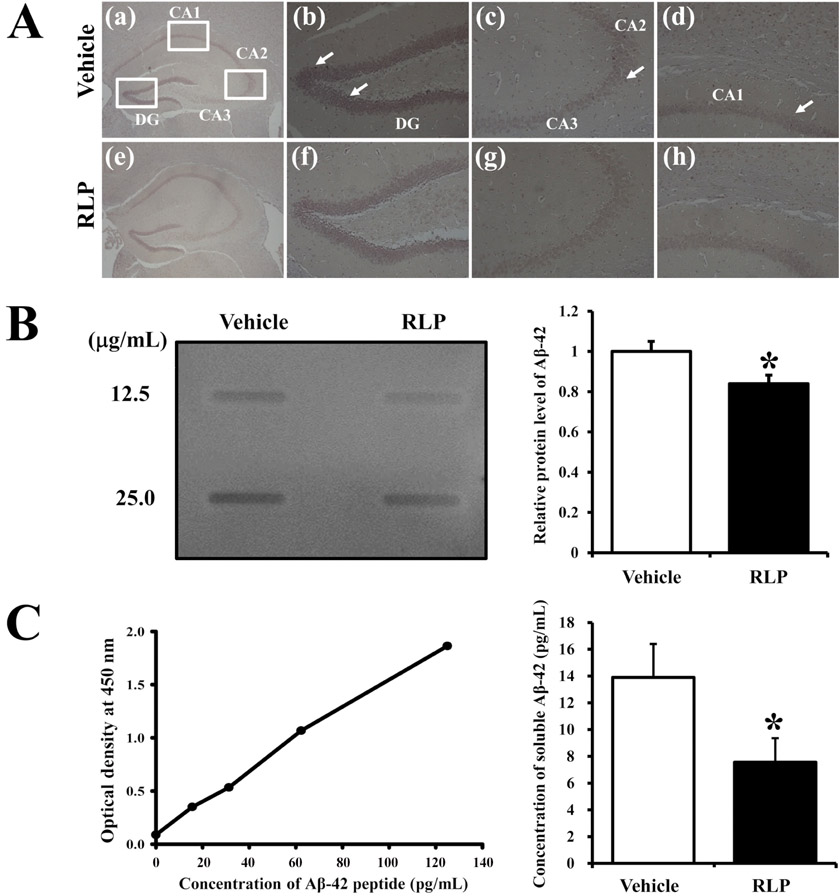
- Liriope platyphylla (LP) has long been regarded as a curative herb for the treatment of diabetes, asthma, and neurodegenerative disorders. To examine the therapeutic effects of Red LP (RLP) manufactured by steaming process on neurodegenerative disorders, significant alteration of the key factors influencing Alzheimer's Disease (AD) was detected in NSE/hAPPsw transgenic (Tg) mice after RLP treatment. The concentration of nerve growth factor (NGF) in serum increased in RLP-treated NSE/hAPPsw Tg mice compared with vehicle-treated Tg mice. However, downstream effectors of the NGF receptor signaling pathway, including TrkA and p75NTR proteins, were suppressed in RLP-treated NSE/hAPPsw Tg mice. Especially, Tg mice showed decreased levels of TrkA, p75NTR, and RhoA expression. Production of Abeta-42 peptides was lower in RLP-treated NSE/hAPPsw Tg mice than in vehicle-treated Tg mice. Further, analysis of gamma-secretase components showed that Abeta-42 peptide expression was downregulated. Of the four components, the expression of APH-1 and Nicastrin (NCT) decreased in RLP-treated NSE/hAPPsw Tg mice, whereas expression of PS-2 and Pen-2 was maintained or increased within the same group. Overall, these results suggest that RLP can help relieve neurodegenerative diseases, especially AD, through upregulation of NGF secretion ability, activation of NGF signaling pathway, downregulation of Abeta-42 peptide deposition, and alteration of gamma-secretase components.
MeSH Terms
-
Alzheimer Disease
Amyloid Precursor Protein Secretases
Animals
Asthma
Down-Regulation
Mice
Mice, Transgenic
Nerve Growth Factor
Neurodegenerative Diseases
Peptides
Proteins
Receptor, Nerve Growth Factor
Steam
Up-Regulation
Amyloid Precursor Protein Secretases
Nerve Growth Factor
Peptides
Proteins
Receptor, Nerve Growth Factor
Steam
Figure
Reference
-
1. Lee YC, Lee JC, Seo YB, Kook YB. Liriopis tuber inhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. nhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. J Ethnopharmacol. 2005. 101(1-3):144–152.2. Choi SB, Wha JD, Park S. The insulin sensitizing effect of homoisoflavone-enriched fraction in Liriope platyphylla Wang et Tang via PI3-kinase pathway. Life Sci. 2004. 75(22):2653–2664.3. Jeong S, Chae K, Jung YS, Rho YH, Lee J, Ha J, Yoon KH, Kim GC, Oh KS, Shin SS, Yoon M. The Korean traditional medicine Gyeongshingangjeehwan inhibits obesity through the regulation of leptin and PPARalpha action in OLETF rats. J Ethnopharmacol. 2008. 119(2):245–251.4. Kim SW, Chang IM, Oh KB. Inhibition of the bacterial surface protein anchoring transpeptidase sortase by medicinal plants. Biosci Biotechnol Biochem. 2002. 66(12):2751–2754.5. Lee YK, Kim JE, Nam SH, Goo JS, Choi SI, Choi YH, Bae CJ, Woo JM, Cho JS, Hwang DY. Differential regulation of the biosynthesis of glucose transporters by the PI3-K and MAPK pathways of insulin signaling by treatment with novel compounds from Liriope platyphylla. Int J Mol Med. 2011. 27(3):319–327.6. Hur J, Lee P, Kim J, Kim AJ, Kim H, Kim SY. Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biol Pharm Bull. 2004. 27(8):1257–1260.7. Hur J, Lee P, Moon E, Kang I, Kim SH, Oh MS, Kim SY. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol. 2009. 620(1-3):9–15.8. Choi SI, Park JH, Her YK, Lee YK, Kim JE, Nam SH, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. Effects of Water Extract of Liriope platyphylla on the mRNA Expression and Protein Secretion of Nerve Growth Factors. Korean J Med Crop Sci. 2010. 18:291–297.9. Kim K, Kim HY. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008. 120(2):190–195.10. Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009. 7(3):293–302.11. Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng). J Pharm Pharmacol. 2006. 58(8):1007–1019.12. Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003. 68(8):1539–1542.13. Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996. 62(1):86–87.14. Yun TK, Lee YS, Kwon HY, Choi KJ. Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao. 1996. 17(4):293–298.15. Choi SI, Lee HR, Goo JS, Kim JE, Nam SH, Hwang IS, Lee YJ, Prak SH, Lee HS, Lee JS, Jang IS, Son HJ, Hwang DY. Effects of Steaming Time and Frequency for Manufactured Red Liriope platyphylla on the Insulin Secretion Ability and Insulin Receptor Signaling Pathway. Lab Anim Res. 2011. 27(2):117–126.16. Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song CW, Paik SG, Cho JS, Kim YK. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. 2002. 16(8):805–813.17. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010. 1327:118–124.18. Torres KC, Dutra WO, Gollob KJ. Endogenous IL-4 and IFN-gamma are essential for expression of Th2, but not Th1 cytokine message during the early differentiation of human CD4+ T helper cells. Hum Immunol. 2004. 65(11):1328–1335.19. Wang YJ, Pollard A, Zhong JH, Dong XY, Wu XB, Zhou HD, Zhou XF. Intramuscular delivery of a single chain antibody gene reduces brain Abeta burden in a mouse model of Alzheimer's disease. Neurobiol Aging. 2009. 30(3):364–376.20. Chao MV. The p75 neurotrophin receptor. J Neurobiol. 1994. 25(11):1373–1385.21. Tabuchi M, Yamaguchi T, Iizuka S, Imamura S, Ikarashi Y, Kase Y. Ameliorative effects of yokukansan, a traditional Japanese medicine, on learning and non-cognitive disturbances in the Tg2576 mouse model of Alzheimer's disease. J Ethnopharmacol. 2009. 122(1):157–162.22. Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011. 117(3):388–402.23. Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001. 58(8):1045–1053.24. Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001. 11(3):281–286.25. Tsui-Pierchala BA, Ginty DD. Characterization of an NGF-P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci. 1999. 19(19):8207–8218.26. Iwatsubo T. The gamma-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol. 2004. 14(3):379–383.27. Vassar R. BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci. 2004. 23(1-2):105–114.28. Hwang DY, Cho JS, Lee SH, Chae KR, Lim HJ, Min SH, Seo SJ, Song YS, Song CW, Paik SG, Sheen YY, Kim YK. Aberrant expressions of pathogenic phenotype in Alzheimer's diseased transgenic mice carrying NSE-controlled APPsw. Exp Neurol. 2004. 186(1):20–32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Precautionary effects of Red Liriope platyphylla on NGF secretion and Abeta42 deposition under the preclinical stage of Alzheimer's disease in Tg2576 mice
- In vitro and in vivo study of effects of fermented soybean product (chungkookjang) on NGF secretion ability and NGF receptor signaling pathway
- Overexpression of N141I PS2 increases γ-secretase activity through up-regulation of Presenilin and Pen-2 in brain mitochondria of NSE/hPS2m transgenic mice
- Effects of Steaming Time and Frequency for Manufactured Red Liriope platyphylla on the Insulin Secretion Ability and Insulin Receptor Signaling Pathway
- Norepinephrine/β2 -Adrenergic Receptor Pathway Promotes the Cell Proliferation and Nerve Growth Factor Production in Triple-Negative Breast Cancer