Lab Anim Res.
2014 Jun;30(2):54-63. 10.5625/lar.2014.30.2.54.
Toxicity of fermented soybean product (cheonggukjang) manufactured by mixed culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on liver and kidney of ICR mice
- Affiliations
-
- 1College of Natural Resources & Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Experimental Animal Research, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3College of Human Ecology, Pusan National University, Busan, Korea.
- KMID: 1707444
- DOI: http://doi.org/10.5625/lar.2014.30.2.54
Abstract
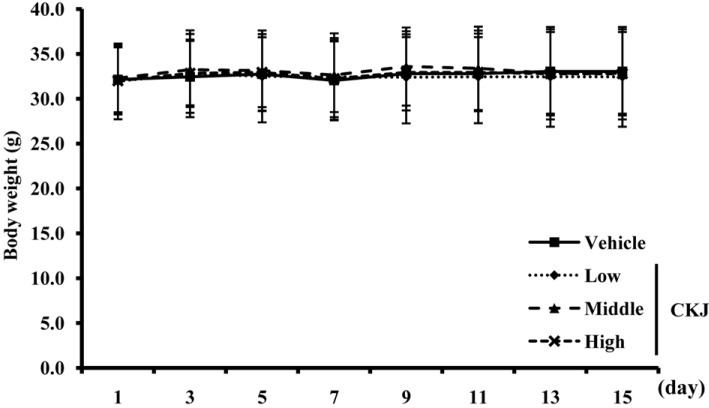
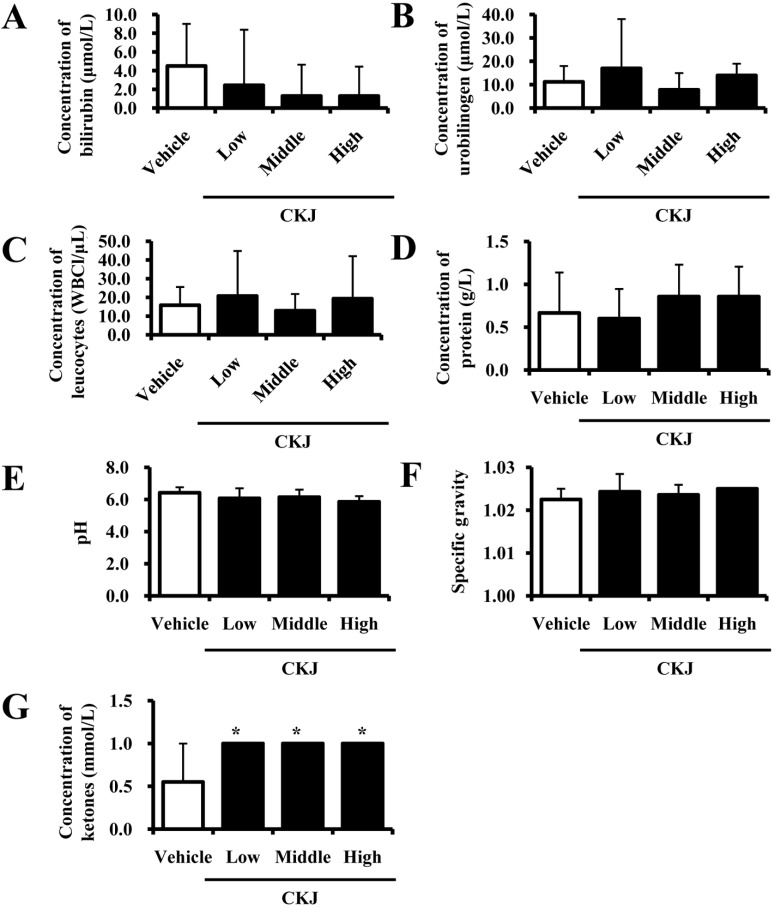
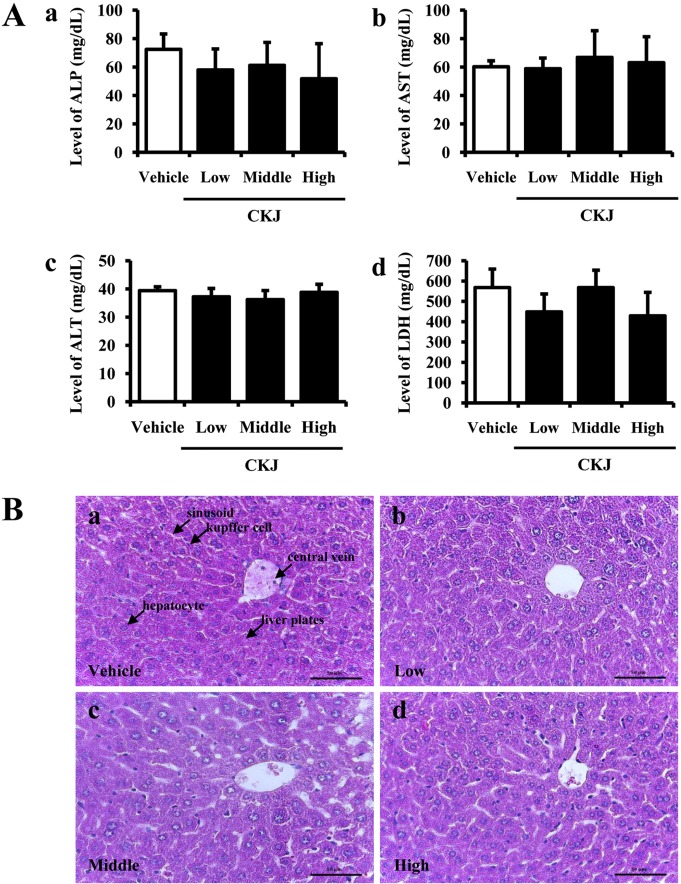
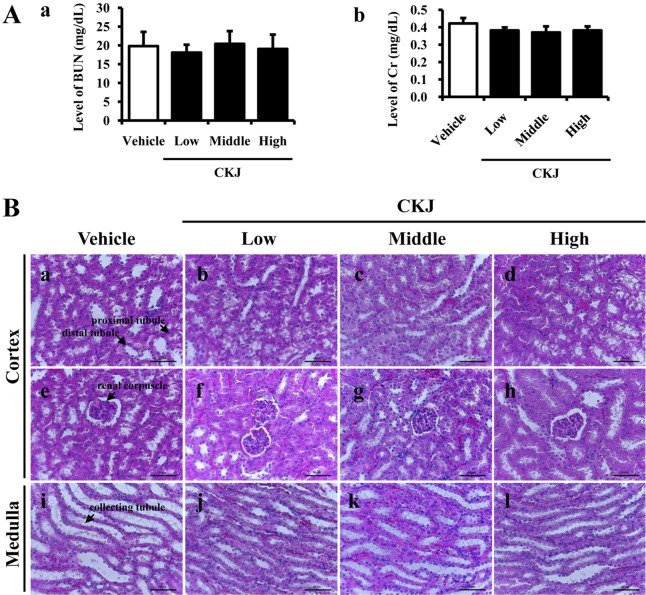
- To investigate the toxic effects of cheonggukjang (CKJ) manufactured using mixed cultures of Bacillus subtilis MC31 and Lactobacillus sakei 383 on the liver and kidney of ICR mice, an alteration on the related markers including body weight, organ weight, urine composition, liver pathology and kidney pathology were analyzed after oral administration at dosage of 25, 50 and 100 mg/kg body weight/day of CKJ for 14 days. Any significant toxicity was not observed on the body and organ weight, clinical phenotypes, urine parameters and mortality in the CKJ-treated group compared with the vehicle-treated group. Also, liver toxicity analysis revealed no significant increase in alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) or lactate dehydrogenase (LDH) in response to CKJ. Additionally, the specific pathological features induced by most toxic compounds were not observed upon liver histological analysis. Furthermore, kidney toxicological analysis revealed that blood urea nitrogen (BUN) and the serum creatinine (Cr) levels and pathological features on histological sections did not differ significantly between the vehicle- and CKJ-treated groups. Overall, these results suggest that CKJ does not induce any specific toxicity in liver and kidney organs of ICR at dose of 100 mg/kg body weight/day as no observed adverse effect level (NOAEL).
MeSH Terms
-
Administration, Oral
Alanine Transaminase
Alkaline Phosphatase
Animals
Aspartate Aminotransferases
Bacillus subtilis*
Blood Urea Nitrogen
Body Weight
Creatinine
Kidney*
L-Lactate Dehydrogenase
Lactobacillus*
Liver*
Mice
Mice, Inbred ICR*
Mortality
No-Observed-Adverse-Effect Level
Organ Size
Pathology
Phenotype
Soybeans*
Alanine Transaminase
Alkaline Phosphatase
Aspartate Aminotransferases
Creatinine
L-Lactate Dehydrogenase
Figure
Cited by 1 articles
-
Comparison of laxative effects of fermented soybeans (
Cheonggukjang ) containing toxins and biogenic amines against loperamide-induced constipation mouse model
Ha-Rim Kim, In-Sun Park, Su-Bin Park, Hee-Jong Yang, Do-Youn Jeong, Seon-Young Kim
Nutr Res Pract. 2022;16(4):435-449. doi: 10.4162/nrp.2022.16.4.435.
Reference
-
1. Lee JJ, Lee DS, Kim HB. Fermentation patterns of chungkookjang and Kanjang by Bacillus licheniformis B1. Korean J Microbiol. 1999; 35(4):296–301.2. Su CL, Wu CJ, Chen FN, Wang BJ, Sheu SR, Won SJ. Supernatant of bacterial fermented soybean induces apoptosis of human hepatocellular carcinoma Hep 3B cells via activation of caspase 8 and mitochondria. Food Chem Toxicol. 2007; 45(2):303–314. PMID: 17030378.
Article3. Nakajima N, Nozaki N, Ishihara K, Ishikawa A, Tsuji H. Analysis of isoflavone content in tempeh, a fermented soybean, and preparation of a new isoflavone-enriched tempeh. J Biosci Bioeng. 2005; 100(6):685–687. PMID: 16473782.
Article4. Kwon DY, Jang JS, Lee JE, Kim YS, Shin DH, Park S. The isoflavonoid aglycone-rich fractions of Chungkookjang, fermented unsalted soybeans, enhance insulin signaling and peroxisome proliferator-activated receptor-gamma activity in vitro. Biofactors. 2006; 26(4):245–258. PMID: 17119271.5. Lee SJ, Rim HK, Jung JY, An HJ, Shin JS, Cho CW, Rhee YK, Hong HD, Lee KT. Immunostimulatory activity of polysaccharides from Cheonggukjang. Food Chem Toxicol. 2013; 59:476–484. PMID: 23831309.
Article6. Back HI, Kim SR, Yang JA, Kim MG, Chae SW, Cha YS. Effects of Chungkookjang supplementation on obesity and atherosclerotic indices in overweight/obese subjects: a 12-week, randomized, double-blind, placebo-controlled clinical trial. J Med Food. 2011; 14(5):532–537. PMID: 21434780.7. Soh J, Kwon DY, Cha YS. Hepatic gene expression profiles are altered by dietary unsalted Korean fermented soybean (chongkukjang) consumption in mice with diet-induced obesity. J Nutr Metab. 2011; 2011:260214. PMID: 21437188.
Article8. Kim DJ, Jeong YJ, Kwon JH, Moon KD, Kim HJ, Jeon SM, Lee MK, Park YB, Choi MS. Beneficial effect of chungkukjang on regulating blood glucose and pancreatic beta-cell functions in C75BL/KsJ-db/db mice. J Med Food. 2008; 11(2):215–223. PMID: 18598161.9. Kwon DY, Daily JW 3rd, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010; 30(1):1–13. PMID: 20116654.
Article10. Choi YH, Lim H, Heo MY, Kwon DY, Kim HP. Anti-inflammatory activity of the ethanol extract of Chungkukjang, Korean fermented bean: 5-lipoxygenase inhibition. J Med Food. 2008; 11(3):539–543. PMID: 18800904.
Article11. Kim HB, Lee HS, Kim SJ, Yoo HJ, Hwang JS, Chen G, Youn HJ. Ethanol extract of fermented soybean, Chungkookjang, inhibits the apoptosis of mouse spleen, and thymus cells. J Microbiol. 2007; 45(3):256–261. PMID: 17618232.12. Lee YJ, Kim JE, Kwak MH, Go J, Son HJ, Kim DS, Hwang DY. In vitro and in vivo study of effects of fermented soybean product (chungkookjang) on NGF secretion ability and NGF receptor signaling pathway. Lab Anim Res. 2013; 29(2):113–126. PMID: 23825484.13. Hwang IS, Kim JE, Lee YJ, Kwak MH, Lee HG, Kim HS, Lee HS, Hwang DY. Growth sensitivity in the epiphyseal growth plate, liver and muscle of SD rats is significantly enhanced by treatment with a fermented soybean product (cheonggukjang) through stimulation of growth hormone secretion. Mol Med Rep. 2014; 9(1):166–172. PMID: 24173540.
Article14. Wongputtisin P, Khanongnuch C, Khongbantad W, Niamsup P, Lumyong S. Screening and selection of Bacillus spp. for fermented corticate soybean meal production. J Appl Microbiol. 2012; 113(4):798–806. PMID: 22788990.
Article15. Fujita H, Yamagami T. Absence of mutagenicity, genotoxicity, and subchronic oral toxicity of Touchi extract. Int J Toxicol. 2007; 26(5):465–473. PMID: 17963133.
Article16. Kim JG. Antigenotoxic effects of water extract from Korean fermented soybean paste (doen-jang). J Food Prot. 2004; 67(1):156–161. PMID: 14717366.
Article17. Zhang G, Bown AW. The rapid determination of γ-aminobutyric acid. Phytochemistry. 1997; 44(6):1007–1009.
Article18. Choi YH, Lim H, Heo MY, Kwon DY, Kim HP. Anti-inflammatory activity of the ethanol extract of Chungkukjang, Korean fermented bean: 5-lipoxygenase inhibition. J Med Food. 2008; 11(3):539–543. PMID: 18800904.
Article19. Kim JH. Protective effects of purple sweet potato added to Bacillus subtilis-fermented soymilk against amyloid beta-induced memory impairment. J Agric Sci. 2012; 4(4):223–232.
Article20. Bae MJ, Shin HS, See HJ, Chai OH, Shon DH. Cheonggukjang ethanol extracts inhibit a murine allergic asthma via suppression of mast cell-dependent anaphylactic reactions. J Med Food. 2014; 17(1):142–149. PMID: 24456365.
Article21. Yang SO, Chang PS, Lee JH. Isoflavone distribution and â-glucosidase activity in cheonggukjang, a traditional Korean whole soybean-fermented food. Food Sci Biotechnol. 2006; 15(1):96–101.22. Kindoli S, Lee HA, Kim JH. Properties of Bac W42, a bacteriocin produced by Bacillus subtilis W42 isolated from Cheonggukjang. J Microbiol Biotechnol. 2012; 22(8):1092–1100. PMID: 22713985.
Article23. Lee NK, Yeo IC, Park JW, Kang BS, Hahm YT. Isolation and characterization of a novel analyte from Bacillus subtilis SC-8 antagonistic to Bacillus cereus. J Biosci Bioeng. 2010; 110(3):298–303. PMID: 20547349.
Article24. Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007; 168(2):176–185. PMID: 17197136.
Article25. Yun TK, Lee YS, Kwon HY, Choi KJ. Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao. 1996; 17(4):293–298. PMID: 9812705.26. Ryu KS, Lee HS, Kim SY. Effects of Bombyx mori larvae extracts on carbon tetrachloride-induced hepatotoxicity in mice. J Life Sci. 1999; 9(4):375–381.27. Stripp B, Hamrick ME, Gillete JR. Effect of 3-methylcholanthrene induction on the carbon tetrachloride-induced changes in rat hepatic microsomal enzyme system. Biochem Pharmacol. 1972; 21(5):745–747. PMID: 5021594.
Article28. Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967; 19(2):145–208. PMID: 4859860.29. You AS, Jeong MH, Park KH, Kim BS, Lee JB, Choi JH, Kwon OK, Kim JH. Effect on antioxidant function of onion to reduce pesticides toxicity. Korean J Pestic Sci. 2007; 11(4):222–229.30. Bergmeyer HU. Methods of enzymatic analysis. 2nd ed. Academic Press: Weinheim;1974. p. 20.31. Yoon SH, Park EJ, Oh KH, Chung YG, Kwon OJ. The effect of Lithospermi radix on benzo(a)pyrene-induced hepatotoxicity. J Korean Soc Food Nutr. 1993; 22(2):144–148.32. Askenazi SS, Perlman M. Pulmonary hypoplasia: lung weight and radial alveolar count as criteria of diagnosis. Arch Dis Child. 1979; 54(8):614–618. PMID: 507916.
Article33. Chung JG, Cho YH, Joo SK, Seon HG, Kim EK, Jeong HC, Lee JH. Amiodarone-induced pulmonary toxicity with hemoptysis and a lung mass. Korean J Med. 2009; 77(5):S1202–S1205.34. Bae MJ, Shin HS, See HJ, Chai OH, Shon DH. Cheonggukjang ethanol extracts inhibit a murine allergic asthma via suppression of mast cell-dependent anaphylactic reactions. J Med Food. 2014; 17(1):142–149. PMID: 24456365.
Article35. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999; 15(6):412–426. PMID: 10634967.
Article36. Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, Lambert M, Lapierre P, Potier E. Medical aspects of ketone body metabolism. Clin Invest Med. 1995; 18(3):193–216. PMID: 7554586.37. Inzucchi SE, Sherwin RS. Type 1 diabetes mellitus. In : Goldman L, Schafer AI, editors. Cecil Medicine. 24th ed. Philadelphia: Saunders Elsevier;2011. chap 247.38. Cahill GF Jr, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA Jr, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966; 45(11):1751–1769. PMID: 5926444.
Article39. Terada Y, Eguchi Y, Chang YJ, Tabata R, Sakumoto H, Takehiro O, Uno S, Ozawa K. Ketone body ratios of the superior and inferior vena cava and of pulmonary arterial blood compared to that of arterial blood: central venous ketone body ratio as a substitute for the arterial ketone body ratio. Clin Chim Acta. 1996; 247(1-2):81–88. PMID: 8920229.
Article40. Hayes AW. Principles and methods of toxicology. 2nd ed. New York: Raven Press;1982. p. 447–474.41. Kim DH, Deung YK, Lee YM, Yoon YS, Kwon KR, Park DB, Park YK, Lee KJ. The liver protecting effect of Pomegranate (Punica granatum) seed oil in mice treated with CCl4. Korean J Electron Microsc. 2006; 36(3):173–182.42. Horiguchi H1, Oguma E, Kayama F, Sato M, Fukushima M. Dexamethasone prevents acute cadmium-induced hepatic injury but exacerbates kidney dysfunction in rabbits. Toxicol Appl Pharmacol. 2001; 174(3):225–234. PMID: 11485383.
Article43. Van De Graaff KM. Human anatomy. 6th ed. New York: McGraw-Hill;2002. p. 93–113.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-hyperlipidemic effect of soybean extract fermented by Bacillus subtilis MORI in db/db mice
- Evaluating the Allergenicity of Soybean by the Fermentation
- The Synergistic Effects of Antimicrobial Peptides on the Growth Inhibition of Salmonella Typhimurium through Imd Pathway in Drosophila Intestine
- Comparison of laxative effects of fermented soybeans (Cheonggukjang) containing toxins and biogenic amines against loperamide-induced constipation mouse model
- Study on Aflatoxins in Korean Fermented Foodstuffs